A joint research group led by Assistant Professor Naoki Ousaka and Professor Shigehisa Akine of the Nano Life Science Institute at Kanazawa University and Professor Mark MacLachlan of the University of British Columbia in Canada has successfully constructed a one-handed α-helical structure using peptides that do not contain any chiral amino acids. The achievement is expected to lead to the development of peptide drugs that require a stable α-helical structure. The study results were published in the October 26, 2023 issue of the international journal Nature Communications.
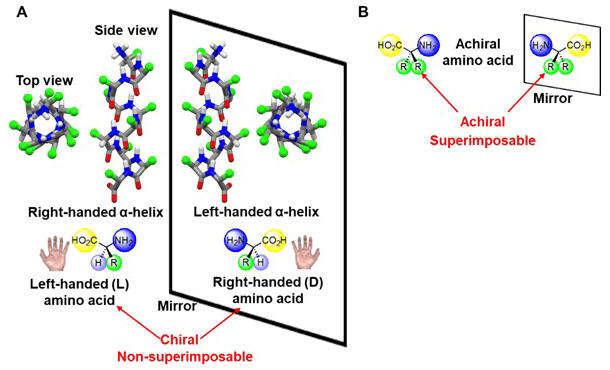
B: An amino acid that is neither right- nor left-handed can be superimposed on its mirror image and is therefore achiral.
© 2023 Ousaka, et al.
Peptides, proteins, and enzymes, which are made up of many amino acids connected together, express their functions by folding into a specific structure. A typical example of such a folded structure is the α-helix.
Natural peptides and proteins are composed of left-handed (L) amino acids and have a right-handed α-helical structure. By contrast, peptides composed of non-natural right-handed (D) amino acids, which have a three-dimensional structure that mirrors the left-handed form, have a left-handed helical structure. Molecules that cannot be superimposed in such a mirror-image relationship are called chiral molecules. Conversely, a molecule that can be superimposed onto its own mirror image is called an achiral molecule.
Those in a mirror-image relationship but cannot be superimposed onto each other, such as the right and left hands, are called mirror-image or optical isomers. The α-helices of peptides that do not contain one-handed (L or D) amino acids should have equal amounts of right- and left-handed bias. In reality, instead of being in equal amounts in solution, the left- and right-handed helices are constantly switching between each other at a rate of several thousand times per second. This meant that it was impossible to remove either the right- or left-handed helices.
In this study, the research group double 'stapled' the sides of the α-helices with 'staple molecules,' which slowed and virtually stopped the interconversion rate by a factor of approximately 1 trillion. This 'stapling' method also allowed for the successful extraction of the one-handed α-helix of the peptide, which does not contain any chiral amino acids.
The researchers predicted that helical inversion could be prevented (slowed down) by 'stapling' the sides of the helical structure with stick-like molecules (staple molecules) that resemble staple needles. The interconversion rate of the single-stapled helical peptides was approximately one million times slower than that of the unstapled peptides.
The double stapling also slowed the interconversion rate by a factor of approximately 1 trillion relative to that of the unstapled peptides. This process almost halted the helix inversion, and an attempt was made to separate the two helices. The right- and left-handed helices were successfully separated using an optical resolution column. It was estimated that returning to the original equilibrium takes several years or more.
Ousaka and Akine said, "Now that the results of this research have demonstrated that we can separate right- and left-handed α-helices, we would like to develop a method to selectively synthesize only one helix without using chiral amino acids in the future. The 'stapling' technique developed in this study can also be used to immobilize various higher-order structures of proteins and is expected to be applied to the development of enzymes that are stable at high temperatures and do not undergo thermal denaturation."
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




