A research group led by Professor Shun Hirota and Graduate Student Takahiro Sakai of the Graduate School of Science and Technology at Nara Institute of Science and Technology (NAIST), in collaboration with the Center for Computational Sciences of the University of Tsukuba, the Institute for Research Management of Oita University, and the Graduate School of Science of the University of Hyogo, announced the discovery of an aggregate where antibodies, which recognize and attack pathogens and other foreign substances through immune reactions, bind in a new pattern. Column chromatography and X-ray crystal structure analyses revealed that the state where four antibody light chains aggregate to form a tetramer is in equilibrium with the monomeric state. The findings are expected to lead to the development of new drugs. The study results were published in the international academic journal Nature Communications on December 8.
Provided by NAIST
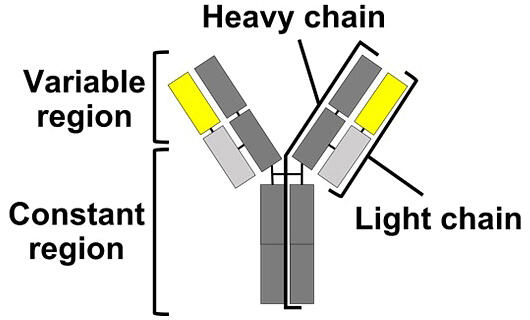
An antibody comprises a set of two long (heavy) and short (light) protein chains (immunoglobulins) that combine to form a "Y" shape, the upper part (variable region) of which recognizes antigens. The light chain is located at the top of the Y shape and contains the variable and constant regions. Drugs based on antibodies are attracting a lot of attention owing to their high therapeutic efficacy and low side effects.
However, antibodies tend to aggregate easily, and when they do so, their ability to recognize antigens is impaired. The accumulation of protein aggregates also causes harm to living organisms. In particular, incorrectly folded antibody light chains are prone to aggregation, which results in further protein aggregation and deposition. This may lead to organ dysfunction due to amyloid light chain amyloidosis.
However, the structure of antibody aggregates at the atomic level remains unknown. If the state of antibody light chain association could be identified, it would help deepen our knowledge for improving the quality of antibody drugs. Accordingly, the research group used column chromatography to analyze the antibody protein size and X-ray crystallography to analyze the structure.
They discovered an antibody light chain that was in equilibrium between the association and dissociation states. They also successfully crystallized the tetrameric sample and identified its molecular structure at the atomic level using synchrotron X-rays at SPring-8, a large synchrotron radiation facility in Japan. As a result, they found that the variable regions in the monomers associate with each other by means of 3D domain swapping, which is a phenomenon in which a part of the same steric structure is exchanged between molecules, to form dimmers, which further dimerize to form tetramers.
Many hydrophobic amino acid residues exist at the interaction interface between dimers after 3D domain swapping. These hydrophobic interactions stabilize the tetramer by preventing the influence of water molecules. The newly discovered aggregate is thought to be effective in stabilizing antibodies. This discovery is expected to lead to the development and design of new antibody drugs that are easier to handle.
Hirota said, "In recent years, antibodies have attracted attention as pharmaceuticals, but the major problem is that their aggregation renders them unusable." Although various "aggregation" modes exist, the mechanism of antibody aggregation at the atomic level remains largely unknown. The results of our research revealed that a phenomenon called 3D domain swapping occurs at the antigen recognition sites to improve the molecular stability. We hope that the information will be useful for the future development of antibody drugs."
Journal Information
Publication: Nature Communications
Title: Structural and Thermodynamic Insights into Antibody Light Chain Tetramer Formation through 3D Domain Swapping
DOI: 10.1038/s41467-023-43443-4
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




