A research group led by Assistant Professor Nobuo Watanabe of the Department of Emergency and Critical Care Medicine and Visiting Professor Sadaki Inokuchi of the Department of General and Acute Medicine, Tokai University School of Medicine, discovered the basic structure of a compound that can selectively interfere with both the podoplanin receptor C-type lectin-like receptor 2 (CLEC-2) and the collagen receptor glycoprotein VI (GPVI) on platelets in humans and mice. This discovery from among approximately 7 million known compounds is unprecedented in the world. These findings are expected to contribute to accelerating the development of drugs for cancer-associated thrombosis, tumor metastasis, and arteriosclerosis-related thrombosis and were published in the journal Thrombosis and Haemostasis.
Provided by Tokai University
Platelets are an important factor of hemostasis that become activated at the site of vessel injury and aggregate to clot the wound. However, platelet aggregation under pathological conditions, such as cancer and arteriosclerosis, blocks blood vessels and causes thrombosis. On the surface of platelets, dozens of receptors exist that trigger a platelet aggregation reaction, including collagen receptors. Antiplatelet drugs used today (e.g. aspirin and clopidogrel) can be widely applied for thrombosis for prophylactical purposes. However, since these drugs inhibit secondary reactions commonly observed downstream of receptor activation, they carry a risk of bleeding due to their non-selectivity. Therefore, the development of antiplatelet drugs that are selective for each receptor type is desired.
Among the receptors present on platelets, CLEC-2 and GPVI are activated by podoplanin and collagen, respectively.
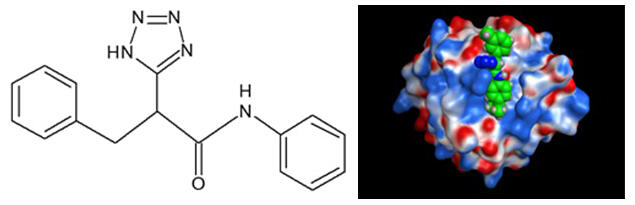
Using their original computer program, the research group performed compound binding assessments of approximately 7 million existing compounds to identify anti-CLEC-2 candidate compounds; approximately 100 compounds were identified for experimental validation. As a result, 12 compounds having the diphenyl-tetrazol-propanamide structure fit both the podoplanin binding site of CLEC-2 and the collagen binding site of GPVI. These compounds inhibited a platelet aggregation reaction in humans and mice by inhibiting interactions between podoplanin and collagen.
However, the drug efficacy was found to be weak in a thrombosis mouse model. The research group clarified that strong binding to albumin in the blood caused this phenomenon, indicating a direction towards basic structural modifications for future clinical application.
Venous thrombosis accounts for approximately 20% of deaths in patients with cancer. The type of venous thrombosis due to aggregation through platelet CLEC-2 activation by podoplanin is partially responsible for these deaths. On the other hand, in arteriosclerosis, when a plaque ruptures, aggregation is induced through the binding of basement membrane collagen to platelet GPVI, which may lead to arterial thrombosis that causes myocardial or cerebral infarction.
Small molecule inhibitors of CLEC-2 or GPVI do not currently exist in the clinical setting or clinical trials. The modification and optimization of the basic diphenyl-tetrazol-propanamide structure discovered by the research group is expected to accelerate the development of effective therapeutic and preventive drugs for cancer-associated thrombosis, tumor metastasis, and arteriosclerosis-related thrombosis.
Journal Information
Publication: Thrombosis and Haemostasis
Title: Diphenyl-tetrazol-propanamide Derivatives Act as Dual-Specific Antagonists of Platelet CLEC-2 and Glycoprotein VI
DOI: 10.1055/a-2211-5202
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




