A research group, led by Assistant Professor Sho Yamaguchi from the Graduate School of Engineering Science at Osaka University, including Graduate Students Daiki Kiyohira (at the time of the research) and Taiki Kawakami, along with Associate Professor Takato Mitsudome and Professor Tomoo Mizugaki, collaborated with Senior Researcher Kohei Tada from the National Institute of Advanced Industrial Science and Technology, and Associate Professor Akira Miura from the Graduate School of Engineering at Hokkaido University. The group successfully synthesized carbon-soluble nickel carbide nanoparticles, a novel variant of nickel nanoparticles widely used in nitrile hydrogenation reactions, leading to a significant enhancement of their catalytic activity. The development of an environmentally friendly, next-generation process is expected for the highly efficient conversion of nitriles to amines using a catalyst composed of relatively inexpensive non-precious metals. The results were published in Chemistry - A European Journal.
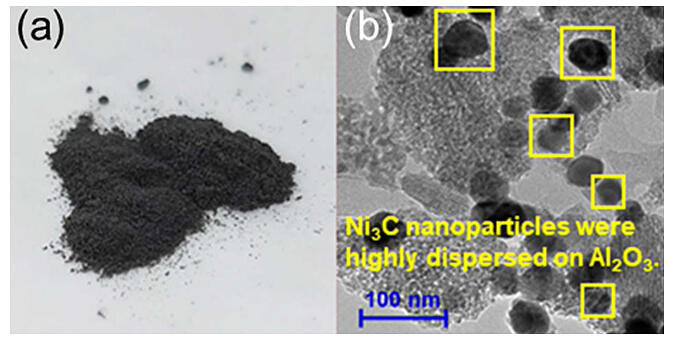
(b) Transmission electron microscope image of nano-Ni3C/Al2O3.
Provided by Osaka University
The hydrogenation of nitriles is an important reaction in the synthesis of important primary amines used as solvents, surfactants, pharmaceutical intermediates and polymer raw materials. Catalyst development using both precious and non-precious metals has been actively conducted. While precious metal catalysts such as palladium and platinum are known for their excellent catalytic activity under mild reaction conditions, they are scarce and expensive.
The research group synthesized nickel carbide nanoparticles (nano-Ni3C) approximately 30 nanometers in size and developed a solid catalyst (nano-Ni3C/Al2O3) where the nano-Ni3C is dispersed and supported on alumina. The solid catalyst exhibits over four times higher catalytic activity in the hydrogenation of nitriles compared to non-carbonized nickel nanoparticles. Moreover, it can selectively convert various types of nitriles into the desired primary amine under ambient hydrogen pressure.
In addition, the catalyst could be easily separated from the reaction solution through centrifugation after the reaction, and no decrease in catalyst activity was observed when the recovered catalyst was reused in subsequent nitrile hydrogenation reactions.
Mizugaki said, "Our research group hopes to contribute to solving the depletion of scarce resources and environmental and energy problems through the development of novel catalysts. In this study, we have developed a catalyst consisting of nanoscale nickel and carbon particles supported on an inorganic material and have shown that the catalyst promotes the hydrogenation of industrially important nitriles very efficiently. It offers a significant improvement in activity compared to the supported nickel nanoparticles widely used in conventional methods and can convert various nitrile compounds to the corresponding primary amines under very mild conditions.
"In addition, the catalyst can be easily reused because the product and catalyst are easily separated after the reaction. We hope that the catalytic reaction process developed through this research, which is energy efficient, safe, and does not emit harmful waste, will contribute to the construction of a sustainable, recycling-oriented, low-carbon society that is environmentally friendly."
Journal Information
Publication: Chemistry - A European Journal
Title: Nickel Carbide Nanoparticle Catalyst for Selective Hydrogenation of Nitriles to Primary Amines
DOI: 10.1002/chem.202303573
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




