A research group led by Assistant Professor Ayumi Nagashima of the School of Life Science and Technology at Tokyo Institute of Technology, in collaboration with researchers at the Faculty of Agriculture of Kindai University, the College of Natural Science of Michigan State University (USA), the Marine Science Museum of Aquamarine Fukushima, and the departments of Physiology and Biomedical Engineering and Nephrology and Hypertension of the Mayo Clinic College of Medicine and Science (USA), announced that they have determined the evolutionary timing where the urea/boric acid transport activity of a specific aquaporin 10 (Aqp10) was diminished in fish, which contain multiple aqp10 genes. The findings are expected to lead to the clarification of new transport substrates and the control of drug transport. The study findings were published in the January 2024 edition of the international academic journal Genome Biology and Evolution (This research was selected as the cover image of Volume 16, Issue 1).
Provided by Tokyo Institute of Technology
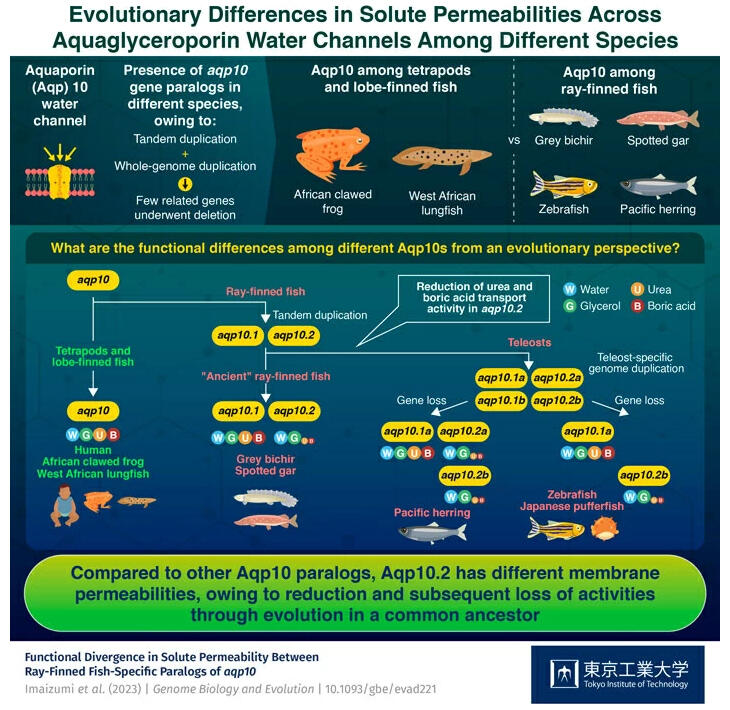
Aquaporins, which are cell membrane proteins, contain two subclasses: the narrow-specificity Aqp, that transports only water, and broad-specificity aquaglyceroporin that transports water and uncharged low-molecular-weight compounds such as glycerol and urea. Aquaglyceroporin is important for transporting these compounds in living organisms. To date, 13 types of Aqp (Aqp0−Aqp12) have been reported in mammals, with Aqp3, 7, 9, and 10 classified as aquaglyceroporins. Amphibians, reptiles, birds, mammals, and their ancestors (lobe-finned fish), have only one aqp10 gene, whereas most fish (ray-finned fishes) have two or more aqp10 paralogs (genes created through tandem duplication).
The research group had previously revealed that human Aqp10 transports water, glycerol, urea, and boron. By contrast, Aqp10.2b, a paralog of tiger pufferfish Aqp10, does not transport urea and boron. However, it was unclear which transport activity was the ancestral type and when the substrate selectivity of the protein had changed during the evolutionary process.
In the present study, the research group obtained the aqp10 genes from lobe-limbed fish (lungfish), humans, ancient ray-finned fishes (gray bichir and spotted gar), and teleosts (Pacific herring, zebrafish, and tiger puffer), expressed them in Xenopus oocytes, and observed the transportation activity of the translated proteins. As a result, they found that transporting urea, boric acid, water, and glycerol is a common ancestral trait (feature), whereas transporting only water and glycerol is found solely in the Aqp10.2 protein of ray-finned fishes.
These results indicate that the urea and boric acid transport activities of Aqp10.2 in ray-finned fishes may have been diminished during the evolutionary process. Although the mechanism of how Aqp10 developed selectivity for substrates other than water remains unclear, the research group's systematic sorting of the differences between the different aqp10 orthologs (a group of genes that diverged during speciation) and among ray-finned fish species-specific paralogs has made future comparisons of the amino acid sequences and activities of the proteins possible.
Nagashima commented, "This result raises many further questions. Do the two types of Aqp10 exhibiting different transport substrate selectivity in ray-finned fishes divide the physiological roles? What kind of activity is found in other organisms that we did not analyze this time? Furthermore, what mechanism causes the difference in transport substrate selectivity between Aqp10.1 and Aqp10.2? I am excited by the thought that, by continuing to answer these questions one by one, I may be able to contribute to the elucidation of the substrate selection mechanism of the entire aquaglyceroporin family in the future."
Journal Information
Publication: Genome Biology and Evolution
Title: Functional Divergence in Solute Permeability between Ray-Finned Fish-Specific Paralogs of aqp10
DOI: 10.1093/gbe/evad221
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




