A research group led by Professor Norio Shibata of the Department of Nanopharmaceutical Sciences and Engineering at the Nagoya Institute of Technology, has announced the successful development of an innovative synthetic method for a group of SF4 acetylene compounds with low environmental impact that could potentially replace PFAS. They applied radical reactions using copper (Cu) catalysts and aromatic diazonium salts. Various SF4 acetylene derivatives could be synthesized in a short time. The obtained SF4 acetylene compounds were also confirmed to be capable of molecular transformation to a more complex group of SF4 compounds. It is expected to lead to the development of alternatives to PFAS. The results were published in the international academic journal Advanced Science on December 31.
Provided by Nagoya Institute of Technology
In recent years, regulations on organo-fluorine compounds have been expanding due to environmental concerns. When fluorine is introduced to organic molecules, additional chemical stability is imparted. Therefore, organo-fluorine compounds are widely used, ranging from pharmaceuticals to everyday products such as frying pans.
On the other hand, highly fluorinated PFOS and PFOA are regulated by the Stockholm Convention because they do not decompose easily when released into the natural environment and remain and accumulate in living organisms.
In this study, the research group focused on SF4 compounds, which consist of four fluorines (F) bonded to a hexavalent sulfur atom (S). This compound exhibits properties similar to PFAS when incorporated to organic compounds. However, it is an organo-fluorine compound exempt from PFAS regulations, lacking the fluoroalkyl moieties that contribute to persistence, thereby demonstrating potential to be utilized as environmentally friendly alternatives to PFAS.
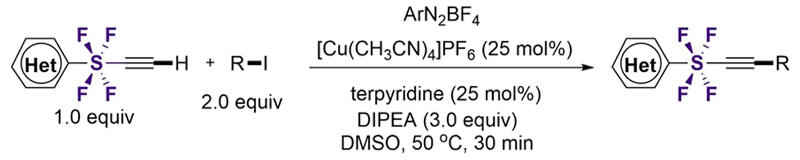
The developed synthetic method is based on a radical cross-coupling reaction. The SF4 acetylene with terminal hydrogen reacts with alkyl iodide in the presence of a Cu catalyst, organic base, ligand, and aromatic diazonium salt. As a result, within 30 minutes of reaction time, SF4 acetylene undergoes efficient cross-coupling with alkyl iodide, leading to the formation of novel SF4 acetylene compounds equipped with various functional groups at the terminal.
The substrate adaptability of this molecular transformation method is very broad, and the transformation can be performed with primary, secondary, and tertiary alkyl iodides. This method is also highly compatible with functional groups such as heterocycles, carbonyl groups, alcohols, and olefins, and its broad substrate adaptability has been demonstrated in over 50 examples. The SF4 compounds obtained through these methods exhibit favorable characteristics as environmentally adaptive agrochemical candidates, thus presenting various potential applications.
Shibata stated, "Developing environmentally friendly alternatives to PFAS is an urgent task. We have developed decomposable SF4 compounds with properties similar to PFAS. We believe that this compound plays a crucial role as a substitute for PFAS, and hope that researchers and companies currently using PFAS will find interest in our research."
Journal Information
Publication: Advanced Sciences
Title: Expanding the Frontier of Linear Drug Design: Cu-Catalyzed Csp-Csp3-Coupling of Electron-Deficient SF4-Alkynes with Alkyl Iodides
DOI: 10.1002/advs.202306554
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




