A research group led by Program-Specific Assistant Professor Tong Zhu, Associate Professor Hiroshi Takatsu, Associate Professor Cédric Tassel, and Professor Hiroshi Kageyama of the Graduate School of Engineering at Kyoto University, in collaboration with Northwestern University, Tohoku University, Osaka University, and the Institute for Materials Structure Science (IMSS) at the High Energy Accelerator Research Organization (KEK), announced the discovery of perovskite chlorides that exhibit multiferroic properties along with temperature-controlled ferroelectric and ferromagnetic properties. The materials with both ferroelectric and ferromagnetic properties reported to date have primarily been oxides. However, this research demonstrated that these properties can also be realized in chlorides. Furthermore, it was newly revealed that the properties of this chloride can be controlled by temperature. These results are expected to remarkably expand the scope of material development and were published in the January 5 issue of the international academic journal Nature Materials.
Provided by Kyoto University
Research on multiferroic materials with both ferromagnetic and ferroelectric properties has been progressing at an accelerated pace in recent years given their potential application to next-generation information storage devices. However, most multiferroic materials developed thus far have been oxides.
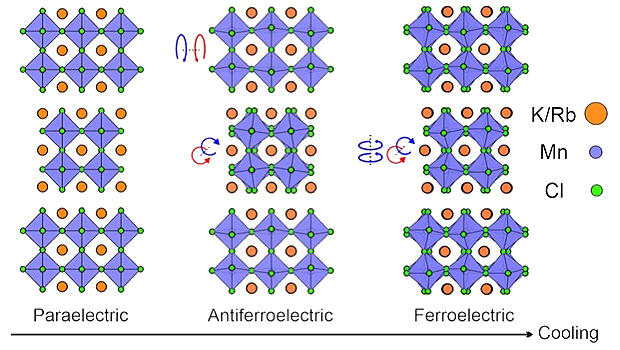
In this study, the research group focused on the chloride (K1-x/Rbx)3Mn2Cl7, which has a perovskite structure. Chloride ions were considered in this study because of their low polarizability, strong ionic properties, and flexibility. The perovskite structure has a layered composition wherein the blocks appear to be stacked on top of each other. Alkali metal ions, primarily K and Rb, dominate the size of the structure of the aforementioned chloride. These ions are surrounded by octahedral MnCl6 units consisting of chloride (Cl) ions and magnetic Mn ions. These octahedra exhibit rotation and tilting within the layer. This property was also considered advantageous for the exhibition of dielectric and magnetic characteristics.
An analysis, which combined neutron and X-ray diffraction, polarization measurements, and theoretical calculations, confirmed that MnCl6 behaves as a paraelectric material (in a state of no polarity) at high temperatures. However, as the temperature decreases, MnCl6 undergoes rotational changes, ultimately transitioning into a ferroelectric state.
A ferromagnetic state was also observed at low temperatures, namely, below 65 K (-208 ℃). Furthermore, by changing the ratio of K to Rb in the aforementioned chloride, the transition temperature (Tc) to ferroelectricity can be adjusted below the temperature at which the magnetic polarization of 65 K occurs. Thus, the dielectric and magnetic properties can be simultaneously manipulated by thermal stimulation.
Zhu stated, "The fusion of chemistry and physics has opened up new research, leading to the achievements presented in this study. We envision actively exploring materials that can be applied to devices in the future. Additionally, we aim to expand the science of multiferroics to include chlorides and combine chloride ions with oxygen ions in our ongoing efforts."
Kageyama stated, "There are also many other divalent elements in addition to Mn, and there is the possibility of varying alkali metals such as K and Rb in the chloride. In my laboratory, interesting results have already been obtained. I anticipate further development in the science of perovskite chlorides in the future. Additionally, we not only aim for fundamental research but also plan to advance device development at the same time."
Journal Information
Publication: Nature Materials
Title: Thermal multiferroics in all-inorganic quasi-two-dimensional halide perovskites
DOI: 10.1038/s41563-023-01759-y
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




