A research group including Research Scientist Satoshi Kamiguchi and Group Director Zhaomin Hou at the RIKEN Center for Sustainable Resource Science, Professor Akira Nakayama at the Graduate School of Engineering at the University of Tokyo, and Professor Kenichi Shimizu at the Institute for Catalysis at Hokkaido University announced the successful and sustainable synthesis of ammonia (NH3) from atmospheric nitrogen (N2) molecules at low temperatures by developing a catalyst with metal clusters comprising approximately six atoms incorporated into numerous holes (fine pores). Through this approach, it is possible to synthesize NH3 fuel that does not emit carbon dioxide (CO2) during combustion under mild conditions. This approach is expected to contribute to energy conservation and development of a decarbonized society. The results were published in the online version of the scientific journal Chemical Science on January 22, 2024.
Provided by RIKEN
NH3 is used in large quantities as a raw material for producing fertilizers and chemical products containing nitrogen. Moreover, it has also attracted attention as a fuel that does not emit CO2, a greenhouse gas. NH3 is typically synthesized from N2 molecules and hydrogen (H2) molecules via the Haber-Bosch process using a solid iron (Fe) catalyst. However, this process is conducted under high-pressure and high-temperature conditions.
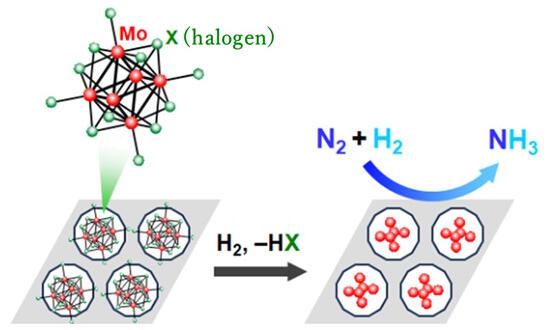
In the proposed synthetic approach, the group removed all halogen ligands via a simple method of incorporating a compound in which halogen is coordinated to a molybdenum (Mo) metal cluster in a porous carrier with fine pores, followed by heat treatment in a H2 atmosphere of 1 atm. They developed a metal-cluster catalyst with a size of < 1 nm. This catalyst can efficiently cleave stable N2 through the synergistic action of multiple Mo atoms and can continuously synthesize NH3 under mild conditions. By utilizing the fine pores, the research group succeeded in suppressing the aggregation of Mo clusters, which reduces reaction efficiency, and efficiently involving Mo atoms in the reaction.
When a mixed gas comprising N2 and H2 was distributed and allowed to react with zeolite that has fine pores accommodating the Mo clusters, NH3 can be generated at a constant rate for approximately 260 h at 400 ℃ under 10 atm and for approximately 520 h even at 200 ℃ under 50 atm.
The as-prepared catalyst can effectively and sustainably cleave N2 at comparatively low temperatures without the use of special components and is safe to handle. In the Fe-catalyzed Haber-Bosch process, the maximum energy is required for breaking the N≡N triple bond. In contrast, Mo has a high cleavage ability, with theoretical calculations suggesting that Mo atoms work cooperatively to realize efficient cleavage. The outcome of this research, particularly the as-prepared ultrafine metal-cluster catalyst, is expected to contribute to the research in the field of nanomaterials and the development of applications.
Kamiguchi stated, "I consider our design of a novel catalyst from a substance not well known as a catalyst material is a significant academic achievement toward the synthesis of high-value-added NH3. Although the synthesis efficiency is still low, we aim to enhance the catalyst activity and optimize the reaction conditions by improving the catalyst properties. In the future, we hope to realize a practical use process integrated with a CO2-emission-free H2 production system based on renewable energy sources."
Journal Information
Publication: Chemical Science
Title: Catalytic ammonia synthesis on HY-zeolite-supported angstrom-size molybdenum cluster
DOI: 10.1039/D3SC05447K
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




