A research group led by Researcher Akiko Yamaguchi and Principal Scientist Masahiko Okumura of the Simulation Technology R&D Office, Center for Computational Science & e-Systems at the Japan Atomic Energy Agency (JAEA), in collaboration with Professor Takashi Yoshimura of the Institute for Radiation Sciences at Osaka University and Professor Yoshio Takahashi of the Graduate School of Science at the University of Tokyo, revealed that the adsorption of metal ions onto clay minerals depends on water solubility and ionic radius. To come to this finding, they analyzed the adsorption behavior through experiments and simulations. This achievement is expected to be useful for not only the recovery of radioactive elements but also metal ion utilization in agriculture and space resource exploration. The results were published in the February 1, 2024 issue of Journal of Colloid and Interface Science.
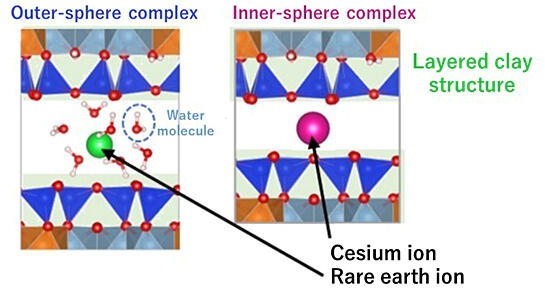
Rare-earth ions adsorbed on clay minerals are surrounded by water molecules and form exospheric complexes, while cesium ions form endospheric complexes without water molecules.
Provided by JAEA
In soil, various metallic elements such as potassium essential for crop growth, rare earth elements utilized as resources, and radioactive substances requiring removal exist in the form of ions adsorbed onto clay minerals. There are two types of adsorption seen in these metal ions. In the first, rare earth elements, surrounded by water molecules, form outer-sphere complexes and are easily extractable. In contrast, radioactive elements, such as cesium, dissociate from water molecules to form inner-sphere complexes and demonstrate strong adherence, thus impeding extraction. However, the factors that determine the formation of outer- or inner-sphere complexes were unknown.
In this study, the research group systematically analyzed the structures of various elements to determine whether they form outer- or inner-sphere complexes when adsorbed onto clay minerals. To conduct the measurement, the researchers employed extended X-ray absorption fine structure (EXAFS) analysis at SPring-8, a large synchrotron radiation facility. The EXAFS analysis allows the examination of atomic arrangements, number of coordinating atoms, interatomic distance, and other factors.
The comparison of the measurement results for various elements revealed that the ions with a large ionic radius form inner-sphere complexes and are strongly adsorbed onto clay minerals. In general, the handling of radium (element symbol: Ra), a radioactive element, has been challenging; detailed information on radium has not been provided. However, it has been revealed that radium forms inner-sphere complexes. Nevertheless, radium was previously considered to form outer-sphere complexes because it is relatively soluble in water.
The research group had developed a method to observe the structure of radium at the molecular level and reported on the dissolution of Ra in water for the first time in 2022. To investigate the significance of ionic radius in the formation of inner- or outer-sphere complexes, they conducted high-precision molecular simulations using a supercomputer. They examined how the stability of clay minerals changes on the basis of the arrangement of metal ions.
They confirmed that the stable arrangement in clay minerals varies with the element. Metal ions undergo adsorption by entering the cavities in the clay minerals surrounding them. For example, the central position of the cavity was stable for potassium adsorption, but for calcium, stable adsorption occurred in an off-center position.
The evaluation of the stability of ions revealed that this difference arises from the force acting between the metal ion and neighboring oxygen atoms, which varies depending on the ionic size. Small ions enter the cavities of the clay minerals along with water molecules, while large ions penetrate the interlayers of clay minerals, thus separating from water molecules. It has been observed that small and large ions form outer-sphere and inner-sphere complexes, respectively.
The experimental and simulation results confirmed that the factors determining the inner- and outer-sphere complexes are correlated to water solubility and ionic size. However, it remained uncertain whether these findings could be applied to metal ions under real world environment. To bridge this knowledge gap, the research group conducted drilling to a depth of 18 m at the Ningyo-Toge Environmental Technology Center of JAEA (Tomata-gun, Okayama Prefecture) to collect borehole samples (soil samples). They analyzed the geology and concentrations of various elements, including Ra, in soil samples. Because this center is located in an old uranium mine, Ra is believed to be present in the soil.
The results indicated that weathered granite with a significant amount of clay minerals contains abundant Ra. Moreover, the trends observed in the experimental and simulation results were reproduced in the actual environment.
This achievement is expected to be widely useful, not only for the safety management of radioactive waste, utilization of radioactive substances, and prevention of contamination, but also for metal ion utilization in agriculture and space exploration.
Yamaguchi stated, "I plan to further my research on radium. Although this experiment utilized representative clay minerals, it is important to note that there are various types of clay minerals. By investigating the correlation between radium and different types of clay minerals or other minerals that coexist with clay minerals, I hope to gain a more detailed and accurate understanding of the behavior of radium in the environment."
Journal Information
Publication: Journal of Colloid and Interface Science
Title: Molecular geochemistry of radium: A key to understanding cation adsorption reaction on clay minerals
DOI: 10.1016/j.jcis.2024.01.120
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




