The research group of Assistant Professor Shun Shibata and Emeritus Professor Takahiro Arima both of the Graduate School of Medicine, Tohoku University, in collaboration with the Institute of Molecular Embryology and Genetics at Kumamoto University, the Institute for Quantitative Biosciences of the University of Tokyo, and the Institute of Biomaterials and Bioengineering of Tokyo Medical and Dental University, has established a method to create an endometrial organoid model using human endometrial cells and succeeded in reproducing each stage of the implantation process. Implantation was reproduced using both a blastoid that mimics a fertilized egg (blastocyst) and the newly developed model. They revealed that the placental cells of the blastocyst fuse with the maternal endometrial stromal cells during the implantation process. A similar phenomenon was confirmed using both human blastocysts and the model during the reproduced implantation. The findings are expected to shed light on the pathology of implantation failure and endometriosis and lead to the development of methods for preventing and treating these conditions. The study results were published on February 23, in the international academic journal Science Advances.
By the time a fertilized egg reaches the endometrium, its cells have increased in number, and the egg has grown. The fertilized egg to be implanted forms a characteristic structure called a blastocyst. Once the blastocyst attaches to the endometrium, pregnancy is established. Implantation will only occur when both the fertilized egg and the uterus are ready. The endometrium is influenced by hormones, with the secretory phase approximately 6−10 days after ovulation is the most conducive for implantation.
It is known that the blastocyst attaches in such a way that the embryo, that will eventually become a fetus, faces toward the endometrial lining. The blastocyst then ruptures the endometrial epithelium and buries itself into the endometrium, adhering to the uterus. However, the mechanism of human implantation remains poorly understood, due to ethical constraints that prohibit observation of this phenomenon. Furthermore, the implantation mechanism of mammals exhibits significant interspecies variations and has not been extensively investigated using laboratory animals. Therefore, the construction of an experimental model was necessary.
In recent years, it has become possible to induce blastocyst-like structures (blastoids) from human cells using embryonic stem (ES) cells and induced pluripotent stem (iPS) cells, and the production of endometrial organoids (miniature organs) has also been reported. However, in the endometrial organoids that have been reported, the uterine membrane epithelium to which an embryo is to be implanted is buried inside, making it difficult to mimic implantation. Subsequently, the research group developed a culture technique to construct an endometrial organoid model for the research.
Initially, utilizing human endometrial tissue and modifying the composition of the culture gel, they demonstrated the feasibility of generating endometrial organoids with the implantation surface positioned externally to the gel. Then, by culturing with other cell types present in the endometrium (endometrial stromal and vascular endothelial cells), they established an endometrial organoid whose spatial arrangement and cell type are similar to those of actual human endometrial tissue. The similarity in gene expression was confirmed through single-cell analysis. They also confirmed that the addition of hormones led to the maturation of the endometrial epithelial cells.
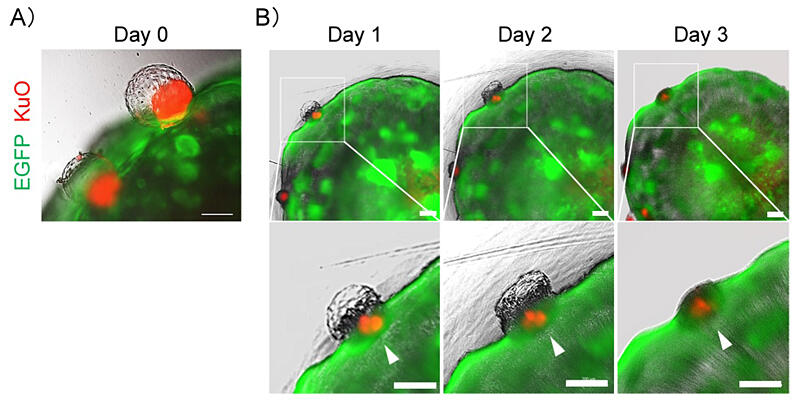
To verify the performance of the model, the research group investigated if it was possible to reproduce implantation using blastoids induced from ES cells. When the endometrial organoid model (fluorescently stained green) and the blastoids (fluorescently stained red) were co-cultured, the blastoids were found to adhere to and burrow inside the endometrial epithelium. Thus, the research group successfully reproduced the infiltration of the fertilized egg into the endometrium.
Upon observing this process through a high-resolution microscope, the researchers witnessed the blastoids destroy the endometrial epithelium and infiltrate the syncytial cells (cells that become the placenta). Additionally, they noted interactions between these cells and internal stromal cells. In the observation, a fine structure of 10 µm could be seen. The syncytial cells were found to infiltrate the endometrial stromal cells over time, and the researchers also succeeded in capturing the fusion of these two cell types for the first time. Furthermore, when human embryos were cultured with the endometrial organoid model, results similar to those obtained with blastoids were obtained.
Shibata said, "In the future, using the endometrial organoid model developed by us as a basis for implantation research, I hope to further clarify the implantation mechanism and to develop methods of infertility treatments targeting cell fusion. Furthermore, I am looking forward to the development of this research, as it may be an excellent model for studying methods of treatments for endometrial disorders."
Journal Information
Publication: Science Advances
Title: Modeling embryo-endometrial interface recapitulating human embryo implantation
DOI:10.1126/sciadv.adi4819
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




