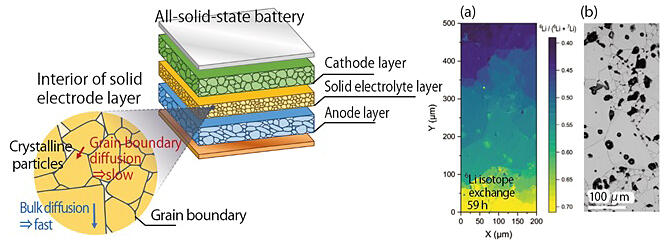
Storage batteries, which support the operation of various electronic devices, such as smartphones and electric vehicles, are an indispensable part of our daily lives. Among them, "all-solid-state batteries," which use a solid electrolyte instead of a liquid one, are attracting attention as a next-generation storage battery that is safer and can achieve higher energy density. In an all-solid-state battery, lithium (Li) ions move between the positive and negative electrodes through a solid electrolyte, generating a flow of electricity. However, resistance caused by Li ion migration at the interface between particles in the electrolyte (grain boundary) is one of the factors contributing to energy density reduction. Therefore, a method that can be used to identify the interface of the ion migration barrier, which is the bottleneck, was needed.
A research group led by Principal Researcher Naoaki Kuwata of the Center for Energy and Environmental Materials at the National Institute for Materials Science has visualized Li ion migration at grain boundaries in solid electrolytes using cryo-SIMS. To do this, the temperature of the sample injected with ions is cooled to −100 ℃ or lower to freeze the movement of the ions while qualitative and quantitative analysis are performed. In this study, a polycrystalline sample of lithium lanthanum titanium oxide was used as a model sample, and 6Li isotopes were introduced at the sample edge by ion exchange. The concentration of 6Li isotopes relative to the total Li concentration (6Li + 7Li) was measured. The results revealed that the 6Li concentration changed significantly at the grain boundaries, i.e., resistance to Li ion was observed at the grain boundaries, and the grain boundaries that prevented Li ion migration were identified.
Using this technique, the migration rate of Li ions in a solid electrolyte was visualized and quantified for the first time in the world. It can be applied to interface measurements of other battery materials and also to the design of interfaces that do not interfere with ion transfer. Furthermore, it is expected to contribute to higher-performance all-solid-state batteries in the future.