A research group led by Project Researcher Linze Xia and Professor Akihito Yamamoto both of the Department of Tissue Regeneration, Institute of Biomedical Sciences in collaboration with Professor Tomonori Iwasaki of the Department of Pediatric Dentistry, Institute of Biomedical Sciences, Professor Eiji Tanaka of the Department of Orthodontics and Dentofacial Orthopedics, Institute of Biomedical Sciences at Tokushima University Graduate School, and Professor Hideharu Hibi of the Department of Oral and Maxillofacial Surgery at Nagoya University Graduate School of Medicine, demonstrated that conditioned medium (CM) of macrophage induced from stem cells can regenerate articular cartilage in a mouse model of osteoarthritis. The findings are expected to lead to safe and low-cost joint regenerative medicine using macrophages obtained from peripheral blood. The study results were published online on February 16 in the American scientific journal Stem Cells Translational Medicine.
Provided by Tokushima University
Patients with osteoarthritis have markedly reduced health levels owing to motor dysfunction and severe pain caused by deformation of the articular cartilage and bones. In Japan, 10 million patients are estimated to have subjective symptoms related to osteoarthritis. In recent years, regenerative medicine that involves harvesting and culturing autologous chondrocytes and synovium-derived mesenchymal stem cells and implanting them into damaged joints has become available, but this treatment is expensive and highly invasive.
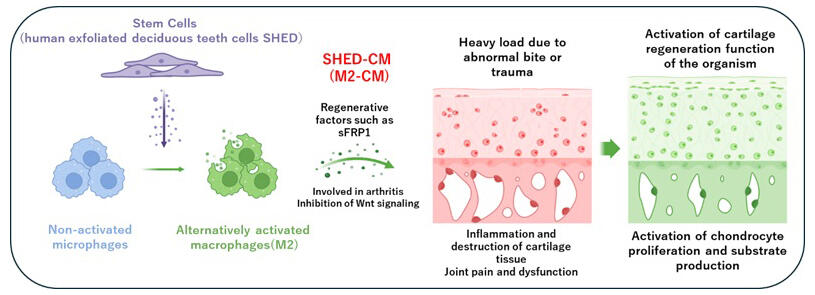
In the current study, the research group found that the administration of CM from stem cells of human exfoliated deciduous teeth (SHED-CM) could suppress articular cartilage damage in mice with temporomandibular joint osteoarthritis (TMJOA) by inducing tissue-repairing macrophages (M2). The research group further demonstrated that the administration of CM of M2 (M2-CM) could suppress inflammatory and matrix-degradation responses in cartilage and enhance chondrocyte proliferation and cartilage matrix regenerative responses.
A comprehensive protein analysis revealed that a Wnt signal antagonist, named secreted frizzled-related protein 1 (sFRP1: a regulator of Wnt signaling), was the major therapeutic factor in M2-CM. The Wnt protein binds to the frizzled receptor on the cell membrane and transmits signals that control cell proliferation and differentiation.
These findings suggest the possibility of using macrophages acquired from peripheral blood for joint regenerative medicine. In contrast to harvesting mesenchymal stem cells (MSCs), which is a process that involves surgery, harvesting macrophages from peripheral blood is much less invasive. Moreover, unlike MSCs, macrophages from peripheral blood do not change with age. By using M2 macrophages induced by embryonic stem cells or induced pluripotent stem (iPS) cells, safe and low-cost regenerative medicine may be achieved.
Yamamoto said, "Because the temporomandibular joint in mice is a small and hard tissue, we spent a great amount of time obtaining reliable experimental results. The first author of this research paper made a persistent effort, with the sole purpose of helping patients suffering from trismus (lockjaw) and managed to publish this study. This study also revealed the importance of Wnt signal control in the regeneration of temporomandibular joint cartilage. In the future, the development of more efficient and safe joint regenerative medicines may be possible by comprehensively analyzing the signals involved in joint regeneration."
Journal Information
Publication: Stem Cells Translational Medicine
Title: Conditioned Medium From Stem Cells of Human Exfoliated Deciduous Teeth Alleviates Mouse Osteoarthritis by Inducing sFRP1-Expressing M2 Macrophages
DOI: 10.1093/stcltm/szae006
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




