A research group led by Professor Kazuya Sasaki of Hirosaki University's Graduate School of Science and Technology Research Division (also at the Lithium Resources Research Organization) and Assistant Professor Kiyoto Shinmura of Hirosaki University Lithium Resources Research Organization announced that they have devised an electrochemical pumping technology that can rapidly and economically extract and recover high-purity lithium from salt lakes, groundwater, and used lithium-ion batteries without any metal impurities. The technology was achieved with two external power sources, three electrodes, and a lithium-ion conductive solid electrolyte membrane. The fast collection and recovery were confirmed to be 464 times faster than similar conventional technologies. In principle, the lithium recovery rate can be infinitely increased. It is expected to contribute towards obtaining lithium resources economically and at an industrial scale. The results were published in the February 11 issue of Communications Engineering.
Provided by Hirosaki University
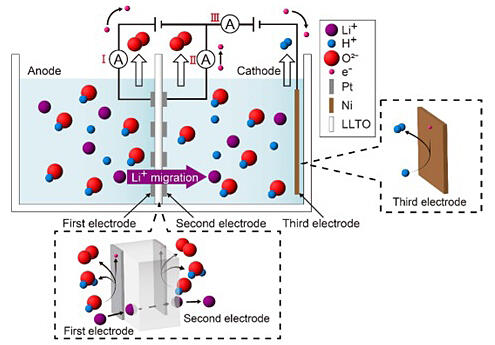
The developed technology enables lithium ions to be transferred to and recovered from the water in the bath on the right (Cathode side) using the electrochemical potential difference between the salt water and the solution of waste LIBs dissolved in the bath on the left (Anode side) of the schematic diagram. Electrochemical pumping technology is a type of technology that provides an electrochemical potential difference between the front and back sides of a diaphragm made of electrolyte that allows a desired ion (in this case the lithium ion Li+) to move inside, thereby moving that ion from one side to the other. Two electrodes formed on the surfaces of both sides of the electrolyte diaphragm generate O2 gas, while the rightmost electrode generates H2 gas, so these gases can also be supplied.
The technology is expected to contribute towards obtaining lithium resources economically at industrial scale for lithium-ion batteries used in electric vehicles and nuclear fusion power generation, which is expected to be a key energy system in the future.
Sasaki said, "Demand for lithium used in lithium-ion batteries for electric vehicles and other purposes is rapidly increasing, and there are concerns for price hikes and supply shortages. Resource recycling will also become mandatory with the enactment of the European Battery Regulation. It will also be used in large quantities in nuclear fusion power generation, which is expected to be the key to energy supply in the future. We are aiming to "create a lithium supply chain" using our technology to ensure a stable and inexpensive supply of lithium, which is indispensable for Japan's economic development and energy security and look forward to the participation of a diverse range of companies."
Journal Information
Publication: Communications Engineering
Title: A three-electrode dual-power-supply electrochemical pumping system for fast and energy efficient lithium extraction and recovery from solutions
DOI: 10.1038/s44172-024-00174-8
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




