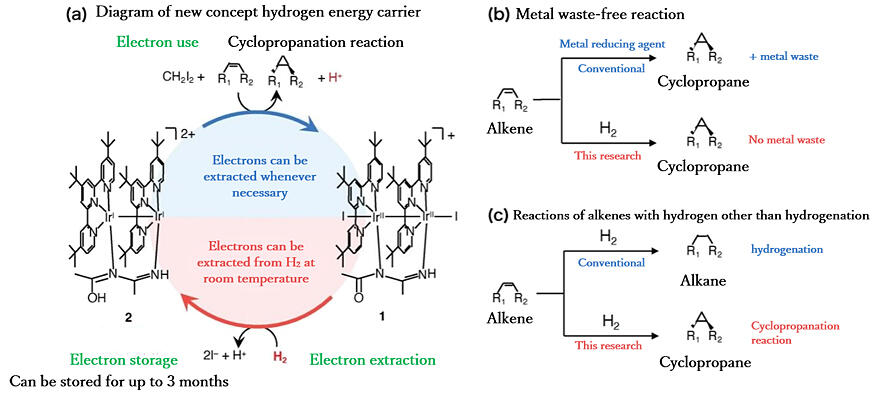
A research group led by Professor Seiji Ogo at the International Institute for Carbon-Neutral Energy Research/Graduate School of Engineering at Kyushu University, in collaboration with Kindai University, has developed a transition metal catalyst (iridium compound) as a hydrogen energy carrier that can extract and store electrons from hydrogen at room temperature and use them in cyclopropanation reactions whenever needed. The catalyst can extract electrons from hydrogen and store them in the solid state for more than three months. Using hydrogen as an electron source allowed for realization of cyclopropanation reactions, which are important for organic and pharmaceutical synthesis, without producing metal waste. The research results were published in the online edition of JACS Au.
b) c) Comparison between conventional reactions and the newly developed one.
Provided by Kyushu University
It is well known that hydrogenation of olefins occurs in the presence of a transition metal catalyst with hydrogen. The research group has been exploring hydrogen energy carriers that can be synthesized from hydrogen at room temperature and used as they are, when compared to natural hydrogenase enzymes that can synthesize and decompose hydrogen at room temperature and has already reported a new nickel-based hydrogen energy carrier. The breakthrough in this research was to use hydrogen as an electron source and extract and store electrons from hydrogen itself rather than from radicals or hydrides.
The research group has begun to develop cyclopropanation reactions, which are important for organic and pharmaceutical synthesis, as a way to utilize hydrogen electrons. At the beginning of the development, however, the reaction progressed slowly. While examining various metal ions and organic ligands, the research group succeeded in developing an iridium compound as a new hydrogen energy carrier. The iridium compound can be synthesized on demand at room temperature on a laboratory scale. Extracted electrons can also be stored in the compound in a solid state at room temperature for more than three months and used for cyclopropanation when needed. Conventional reactions produce metal waste from metal reducing agents, but the proposed reaction uses hydrogen as a reducing agent, thereby resulting in zero metal waste.
Ogo said, "We have developed an energy carrier that extracts electrons from hydrogen and developed a useful organic synthesis reaction that uses hydrogen as the electron source. Because this method is based on a completely different idea from that of conventional ones, I began my paper with the sentence, 'Have you ever imagined reactions of alkenes with hydrogen that result in anything other than hydrogenation or hydrogenative C−C coupling?' We believe that this result will contribute to the realization of a carbon-neutral society."
Journal Information
Publication: JACS Au
Title: Cyclopropanation Using Electrons Derived from Hydrogen: Reaction of Alkenes and Hydrogen without Hydrogenation
DOI: 10.1021/jacsau.4c00098
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




