The research group of Assistant Professor Yuki Yoshino and Professor Natsuko Chiba of the Institute of Development, Aging and Cancer at the Department of Cancer Biology, in collaboration with Graduate Student Tokiwa Motonari and Professor Takanori Ishida of the Graduate School of Medicine at Tohoku University developed a method to determine homologous recombination repair activity in mouse tumor tissue and blood-derived lymphoblastoid cells. They demonstrated that this method could be utilized for predicting the efficacy of cancer drugs and diagnosis of hereditary breast and ovarian cancer syndrome. The study was published in Scientific Reports.
Provided by Tohoku University
PARP inhibitors and platinum-based anticancer drugs are effective against tumors with abnormalities in homologous recombination repair, the process which repairs DNA double-strand breaks. Genetic abnormalities in molecules required for this are known to cause hereditary breast and ovarian cancer syndrome. Therefore, evaluation of homologous recombination repair activity may be useful for predicting the efficacy of cancer therapy and diagnosing hereditary breast and ovarian cancer syndrome.
Until now, genetic testing for factors involved in homologous recombination repair, such as BRCA1 and BRCA2, has been used for clinical evaluation of homologous recombination repair activity. However, genetic testing is ineffective in determining all genes that can cause abnormalities in homologous recombination repair, and the genetic changes found cannot be determined if they are abnormal in some cases.
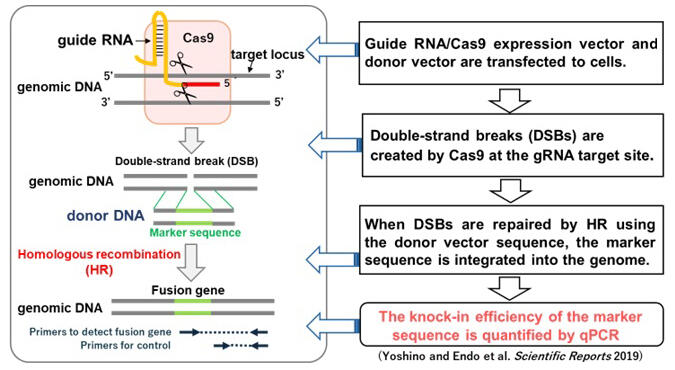
The research group applied ASHRA (Assay for Site-specific HR Activity), a method for measuring homologous recombination repair activity developed by Chiba et al. They succeeded in directly evaluating homologous recombination repair activity in tumor tissue and blood-derived cells. In ASHRA, as the measurement vectors, a Cas9/gRNA expression vector that cleaves genomic DNA, and a donor vector with a sequence homologous to the cleavage site and a marker sequence necessary for detection are introduced into the target cells.
When DNA double-strand breaks in genomic DNA are repaired by homologous recombination repair using the donor vector, the marker sequence is knocked into the DNA double-strand break, resulting in a fusion gene composed of the marker sequence and endogenous gene, which is detected by quantitative PCR. Tumors created by xenografting a human cancer cell line in mice were removed, measurement vectors were injected into the tumor, and electroporation was performed to let the tumor cells incorporate the vectors. Subsequently, the fusion gene generated by homologous recombination repair could be detected. Homologous recombination repair activity measured by this method correlated well with tumor shrinkage when mice were treated with olaparib, a PARP inhibitor. Moreover, homologous recombination repair activity could be detected by introducing the measurement vectors into blood-derived lymphoblastoid cells by electroporation. Furthermore, a significant decrease in homologous recombination repair activity was detected in lymphoblastoid cells from hereditary breast and ovarian cancer syndrome with BRCA1 mutations in one allele.
These findings indicate the possibility that ASHRA-based evaluation of homologous recombination repair activity in blood-derived cells, which are normal cells of patients with hereditary breast and ovarian cancer syndrome, can be applied for diagnosis. As ASHRA can directly detect changes in homologous recombination repair activity, it is likely to successfully detect abnormalities in unknown homologous recombination repair factors as well as in genes of known homologous recombination repair factors that could not be determined whether the changes are abnormal.
Clinical application of this method is expected to improve the effectiveness of cancer treatment and the efficiency of diagnosis of hereditary breast and ovarian cancer syndrome.
Journal Information
Publication: Scientific Reports
Title: Evaluating homologous recombination activity in tissues to predict the risk of hereditary breast and ovarian cancer and olaparib sensitivity
DOI: 10.1038/s41598-024-57367-6
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




