As gold can be utilized as a stable catalyst to promote chemical reactions without changing itself, there are expectations for it to be applied to energy conversion and removal of environmental pollutants. Although it is a metallic element that is inherently less reactive, previous research has shown that gold nanoparticles, which are minute particles with a diameter of 1−100 nm display high reactivity. However, gold nanoparticles tend to aggregate into large particles in solution, making it difficult to maintain stability under catalytic reaction conditions.
A research group led by Associate Professor Kosuke Suzuki and Graduate Student Kang Xia of the School of Engineering at the University of Tokyo has developed gold nanoparticles as small as 3 nm that can remain stable in catalytic reactions in solution. By using "metal oxide nanoclusters," in which multiple metal atoms are bonded via oxygen atoms as a protective agent, they were able to synthesize gold nanoparticles that exhibit excellent catalytic activity.
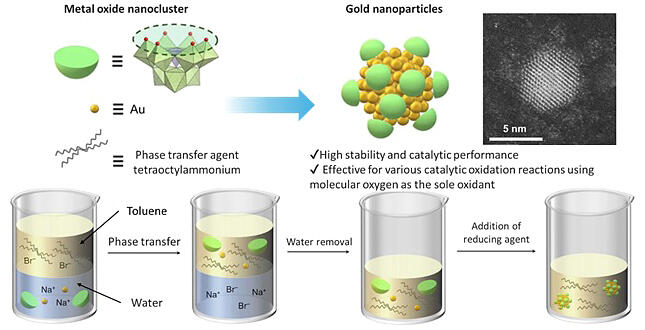
This study uses a two-phase system with water and toluene, which do not mix with each other. Gold ions and metal oxide nanoclusters were dissolved in water, then transferred to toluene with a phase transfer agent, and the reducing agent was added to the toluene with the water removed. As a result, we were able to synthesize gold nanoparticles that exhibit excellent catalytic reactions in solution, which also can maintain high stability with no change in size after one year.
The developed gold nanoparticles act as a catalyst in the oxidation reaction of alcohols to produce aldehydes in yields as high as 90 percent or more. It also shows excellent catalytic properties for oxidation reactions of organic compounds other than alcohols, making it possible to synthesize pharmaceuticals and chemicals under conditions that reduce environmental impact. In addition to gold, nanoparticles can also be synthesized from other precious metals such as ruthenium, rhenium, and rhodium, and are expected to find applications in catalysts, sensors, and semiconductors.