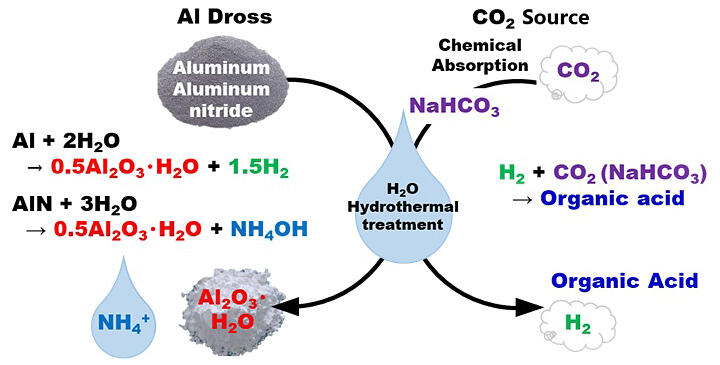
A research group led by Associate Professor Naoto Tsubouchi and Specially Appointed Assistant Professor Yuuki Mochizuki of the Graduate School of Engineering at Hokkaido University, announced that they have successfully produced organic acid salts, such as formate and oxalate, through the hydrothermal treatment of aluminum (Al) dross (dissolved metal) in the presence of CO2 as a CO2 chemical absorption solution. The conversion of metallic and nitrided Al in the dross to boehmite, hydrogen (H2) production, ammonium ion (NH4+) formation, and halogen (chlorine and fluorine) removal were also achieved simultaneously. This is the first time in the world that a carbon-recycling type processing technology has been successfully developed. The results were published in the March 20 issue of ACS Sustainable Chemistry & Engineering.
Provided by Hokkaido University
Demand for aluminum is expected to increase in the automotive industry, which aims to reduce CO2 emissions, owing to its light weight. However, dross is generated as a byproduct of the manufacturing process. This dross is a valuable recycling resource and a difficult waste to process. In Japan, 10% of Al production (370,000 tons per year) is dross. While some of the high Al content dross is effectively used in the steel industry, approximately 30,000 tons per year is disposed of in landfills. The situation is expected to worsen as it is now stored at some production sites due to increasing disposal costs.
Various methods have been studied for detoxification, recycling, and effective utilization of dross; however, dry treatment, which requires high temperatures, generates CO2 and consumes large amounts of energy. The treatment using H2O has the advantage of simultaneous dehalogenation and denitrification, but the nitrogen removal rate was 67% even under hydrothermal conditions at 250℃, which was low and not cost-effective. Additionally, these methods do not consider carbon recycling. Thus, there has been a call for carbon-recycling treatment technologies.
In this study, Al dross was hydrothermally treated in the presence of sodium bicarbonate (NaHCO3), a CO2 source, and analyzed in the gas-liquid solid phase. Through reactions involving metallic Al, H2O, and CO2 in Al dross, H2 and organic acid salts were formed, and metallic Al changed to a boehmite form. It was demonstrated that nitrogen in Al nitride is converted to NH4+, and the nitrogen removal rate from the solid phase reaches about 90%, while halogen is also removed at the same time. The effects of the type of Al dross and the concentration of NaHCO3 were also examined. It was found that the amount of organic acid salts and H2 produced tends to be greater in dross with more metallic Al and an optimal amount of NaHCO3 should be added. In both cases, nitrogen removal reached more than 90% at 300℃, confirming that metallic and nitrided Al are converted to boehmite in this process.
Tsubouchi said, "Our laboratory aims to use the power of chemistry to solve problems related to 'resources, energy, and the environment.' This technology is expected to reduce waste and greenhouse gas emissions because low-grade dross and CO2 are considered as resources to be recycled. Through this kind of research, we hope to contribute to the achievement of the Sustainable Development Goals (SDGs) set by the United Nations."
Journal Information
Publication: ACS Sustainable Chemistry & Engineering
Title: Co-processing Method for the Stabilization of Aluminum Dross and the Production of Valuable Substrates via Hydrothermal Treatment
DOI: 10.1021/acssuschemeng.4c00116
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




