A research group led by Assistant Professor Izumi Sasaki and Professor Tsuneyasu Kaisho of the Department of Physiological Regulation Mechanisms at the Institute of Advanced Medicine of Wakayama Medical University, have clarified the mechanism by which the endoplasmic reticulum (ER) stress sensor IRE1α is required for the production of the inflammatory cytokine IL-1β via inflammasomes using mice. They analyzed the gene expression of cholera toxin-treated murine resident peritoneal macrophages. After examining the response to the toxin, they found that it acts on resident macrophages in the abdominal cavity. After entering the cells via glycolipid gangliosides (GM1) on the cell membrane, the toxin reaches and accumulates in the ER and induces an ER stress response, producing IL-1β. This finding is expected to lead to the development of new inflammation-control drugs for many diseases, and the results were published in the March 22 issue of the international academic journal Cell Reports.
Provided by Wakayama Medical University
Multiple types of inflammasomes are protein complexes composed of a pathogen sensor, a protein-cleaving enzyme, and a molecule that links the two, called the NLRP3 inflammasome or pyrin inflammasome, after each pathogen sensor. The cleavage by this enzyme transforms IL-1β from an inactive to an active form, allowing it to exert its inflammation-inducing activity. Upon pathogen infection, the inflammasome recognizes them using the pathogen senor and facilitates the production of IL-1β from macrophages, which plays an essential role in host defense against infection. On the contrary, it reacts with metabolites in the body, and excessive function leads to the development, or exacerbation of autoinflammatory, diseases and chronic inflammatory diseases such as diabetes mellitus. Genetic mutations in its components have been shown to cause autoinflammatory diseases such as cryopyrin-related periodic fever syndrome and familial Mediterranean fever. Conversely, it has been unclear how inflammasomes are involved in inflammatory pathology, as it is difficult to reproduce them in vivo.
Previously, the group reported that cholera toxin from Vibrio cholerae responds to macrophages residing in the abdominal cavity of mice and induces IL-1β production. They have clarified that the activity of cholera toxin requires entry into the cell via binding to glycolipid gangliosides (GM1) on the cell membrane and subsequent activation of both NLRP3 and the pyrin inflammasome. It was unclear how cholera toxin activates inflammasome. Therefore, in the present study, cholera toxin was added to macrophages residing in the abdominal cavity of mice to perform a comprehensive analysis of the induced gene clusters.
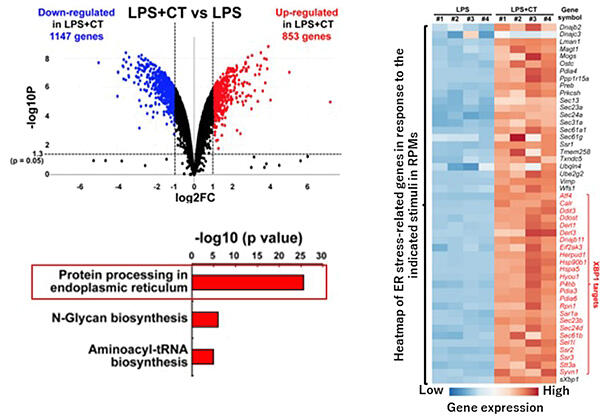
The results showed that 853 genes were upregulated more than twofold upon cholera toxin stimulation, including many genes that induce an ER stress response. The ER stress response is a mechanism that eliminates toxic proteins with abnormal structures. These proteins accumulate in the ER. The degradation or synthesis halt is induced by the activation of ER stress sensors such as PERK and IRE1α, thereby maintaining homeostasis. Immunostaining was performed to examine whether cholera toxin reached the ER of the mouse abdominal cavity macrophages. It was found that cholera toxin colocalized with IRE1α and accumulated. They also examined whether the ER response requires the entry of cholera toxin via binding to GM1 in GM1-deficient macrophages. They found that the toxin did not enter the cell and induced no stress response in GM1-deficient mice. Further investigation using inhibitors to determine whether PERK or IRE1α ER stress sensors are involved showed that only IRE1α is involved in the IL-1β induction, which was also confirmed by experiments using deficient mice. Cholera toxin has been shown to activate both NLRP3 and the pyrin inflammasome. To determine whether IRE1α is involved in either activation, IRE1α-deficient peritoneal macrophages were used to examine their ability to induce IL-1β when each inflammasome activator was added. The IL-1β production capacity was impaired after both additions. It has been clarified that the ER stress sensor IRE1α is required for the activation of both inflammasomes.
Sasaki said, "There are other ER stress sensors, and their roles are still unclear in the field of immunology. We expect that the macrophages residing in the abdominal cavity, which is our focus in this study, will help us better elucidate the molecular basis. Macrophages residing in the abdominal cavity are also present in humans. A similar mechanism is likely to be functioning in humans. We would also like to pursue research with an eye toward human treatment."
Journal Information
Publication: Cell Reports
Title: A stress sensor, IRE1α, is required for bacterial-exotoxin-induced interleukin-1β production in tissue-resident macrophages
DOI: 10.1016/j.celrep.2024.113981
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




