A research group led by Professor Masatomo Yashima, Graduate Students Nachi Ueno and Hiroshi Yaguchi (at the time of the research, now at RIKEN), and Assistant Professor Kotaro Fujii of the Department of Chemistry, School of Science at Tokyo Institute of Technology has announced the discovery of a new group of oxychlorides (substances containing oxygen and chlorine) and other oxyhalides (substances containing oxygen and halogens), which exhibits extremely high oxide ion conductivity and high stability exceeding those of conventional materials. A sufficiently high oxide ionic conductivity was observed at a temperature that is more than 200℃ lower than the temperature of the conventional substance. The result obtained in this research is expected to pave the way for the development of various new materials, including solid oxide fuel cells. The results are published in the April 9 electronic edition of the Journal of the American Chemical Society.
© Authors (2024)
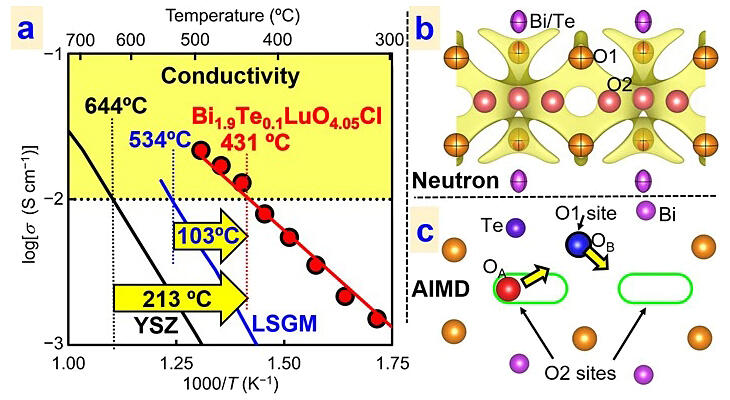
Oxide ion conductors are materials that exhibit oxide ion (O2−) conduction and are expected to be widely applied in solid oxide fuel cells (SOFCs) and oxygen separation membranes. The yttria-stabilized zirconia electrolyte used in current SOFCs requires a high operating temperature (700℃−1000℃), which results in high production costs and degradation at high temperatures. Thus, an oxide ion conductor with high conductivity at moderate temperatures (400℃−500℃) is required. A new oxychloride, Bi2−xTexLuO4+x/2Cl (x = 0.1 and 0.2), was successfully synthesized. This oxide ion conductor is an oxychloride composed of bismuth, tellurium, lutetium, oxygen, and chlorine and is also an oxyhalide. Furthermore, it was found that the composition of the oxychloride "Bi1.9Te0.1LuO4.05Cl" with x = 0.1 has the highest ionic conductivity.
The new oxide ion conductor exhibited an oxide ion conductivity of 10 mS/cm, which is a standard for practical use in fuel cells, at a temperature much lower (431℃) than conventional practical materials (644℃). It was also confirmed that its electrical conductivity is constant in the oxygen partial pressure range of 10−4-10−18 atm and that it is chemically very stable without showing electronic conduction that will reduce power generation efficiency. Moreover, the high oxide ion conductivity is attributed to the two-dimensional diffusion of oxide ions through the triple fluorite-like layer via an interstitialcy mechanism involving interstitialcy and lattice oxygen sites. High ionic conductivity and high stability at medium temperatures of less than 500℃ will lead to the development of low-cost, high-performance electrochemical devices such as SOFCs that operate at medium temperatures.
Yashima said, "Nachi Ueno, who was a graduate student at the time of the research, worked hard to obtain reliable conductivity measurements by performing the equivalent circuit analysis of AC impedance data and was able to demonstrate high oxide ion conductivity. It was also difficult to determine the position of the interstitial oxygen in the crystal structure analysis; however, we were able to clarify a reliable interstitial oxygen position and ionic conduction mechanism. The discovery of a new material that combines high oxide ion conductivity and stability in this research is expected to stimulate research on related materials and lead to the development of high-performance fuel cells with low operating temperatures."
Journal Information
Publication: Journal of the American Chemical Society
Title: High Conductivity and Diffusion Mechanism of Oxide Ions in Triple Fluorite-Like Layers of Oxyhalides
DOI: 10.1021/jacs.4c00265
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




