A research group led by Graduate Students Yoshika Hara (at the time of research), Ken Yoshizawa (at the time of research) and Atsuya Yaguchi (at the time of research) of the Graduate School of Engineering and Specially Appointed Lecturer Noriyuki Uchida and Professor Takahiro Muraoka of Institute of Engineering at Tokyo University of Agriculture and Technology, together with Associate Professor Hirotsugu Hiramatsu of the National Yang Ming Chiao Tung University (Taiwan), has announced that they successfully developed a self-assembling peptide (JigSAP-MMM) that is responsive to oxidative stress and protects cells and tissues from oxidative damage. The peptide is expected to find application in the protection of inflammatory tissues and muscle cells and regenerative medicine. The results were published in the May 9 issue of Biomacromolecules, a journal of the American Chemical Society.
Tokyo University of Agriculture and Technology
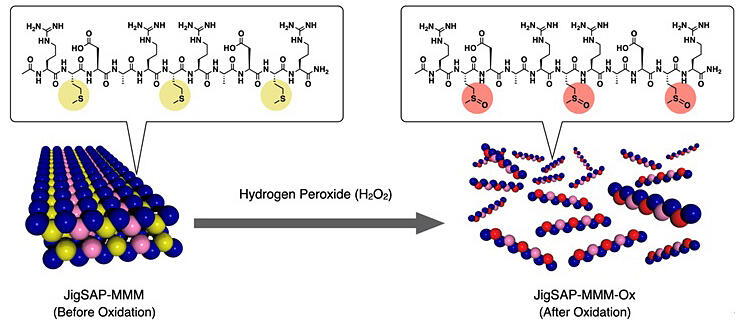
Reactive oxygen species (ROS) are important biological signaling molecules responsible for signal transduction in cellular activities. However, excessive accumulation of these molecules causes cellular oxidative stress, leading to irreversible damage to various biomolecules such as nucleic acids, lipids and proteins, followed by diseases such as type 2 diabetes and cancer. To remove ROS, cells have antioxidant proteins with multiple surface-exposed methionine residues. These antioxidant proteins are known to remove ROS by converting Met to methionine sulfoxide (MetO). In this study, the group synthesized three types of peptides: a self-assembling peptide containing three residues of the hydrophobic amino acid methionine (Met) in the hydrophobic part to impart ROS-responsiveness to the peptide, a peptide containing two Met residues for comparison, and a peptide containing one Met residue for comparison. They evaluated the functions of these peptides.
All three synthesized peptides were confirmed to be capable of self-assembling into supramolecular nanofibers in water to form a hydrogel. When each peptide gel was allowed to react with H2O2 (hydrogen peroxide), a typical ROS, the H2O2-responsiveness of the hydrogel was found to increase with an increase in the number of Met residues placed in the hydrophobic part of the peptide. The self-assembling peptide was also found to effectively prevent cell death due to oxidative stress.
Muraoka commented, "Because ROS are involved in the development of diseases and the progression of aging, the development of antioxidant materials that efficiently remove ROS will contribute widely to health care. In this study, we worked on the development of antioxidant materials based on short-chain peptides exclusively composed of natural amino acids for biological applications. Because our antioxidant peptide is a gel-like material with low fluidity, it stays and functions in the local area for a long time. Peptide gels are also effective as cell scaffolds; hence, we plan to apply the developed material to protect cells and tissues from ROS and to promote tissue regeneration."
Journal Information
Publication: Biomacromolecules
Title: ROS-Responsive Methionine-Containing Amphiphilic Peptides Impart Enzyme-Triggered Phase Transition and Antioxidant Cell Protection
DOI: 10.1021/acs.biomac.4c00129
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




