A research group led by Associate Professor Taku Fujimura of the Department of Dermatology at Tohoku University School of Medicine has conducted a phase II clinical trial of a new treatment for malignant melanoma (melanoma) refractory to conventional immune checkpoint inhibitor combination therapy and achieved a high response rate of 25.9% (protocol per set, PPS) with very few adverse events. The results of their research were published in the British Journal of Dermatology. The new treatment combines a PAI-1 inhibitor developed by Professor Toshio Miyata of Tohoku University and his colleagues with immune checkpoint inhibitors. In collaboration with Renascience, a start-up from Tohoku University, the research group will conduct a phase III clinical trial in 2025 for regulatory approval and commercialization.
Provided by Tohoku University
Malignant melanoma is classified into four types: superficial spreading, acral lentiginous, lentigo maligna, and nodular. The acral lentiginous type accounts for approximately 2% of all cases in the United States and Europe, but 40% in Japan. Three types of immune checkpoint inhibitor therapies, the anti-PD-1 antibodies Opdivo and Keytruda, and Opdivo + Yervoy, are used in first-line therapy; however, the acral lentiginous type is less susceptible to anti-PD-1 antibodies than the superficial spreading type because the former has a lower tumor mutation burden than the latter. In clinical practice, response rates of anti-PD-1 antibodies and Opdivo + Yervoy as first-line therapy have been 16%-22% and 41%, respectively, which are much lower than 38%-44% and 58% in the United States and Europe, highlighting that non-responders to anti-PD-1 antibodies are more common in Japan than in the United States and Europe. Meanwhile, the incidence of grade ≥3 adverse events was 12.5% for Opdivo and 59% for Opdivo + Yervoy. In Japanese anti-PD-1 antibody non-responders, the efficacy of Opdivo + Yervoy combination therapy has been 10-20%, and adverse events requiring hospitalization have occurred in at least 60% of patients. Currently, the standard of care for anti-PD-1 antibody non-responders is Opdivo + Yervoy or Yervoy alone. However, response rates to these therapies in Japanese patients were only 13.5% and 3.6%, respectively, and effective and safe treatments for anti-PD-1 antibody non-responders are still required.
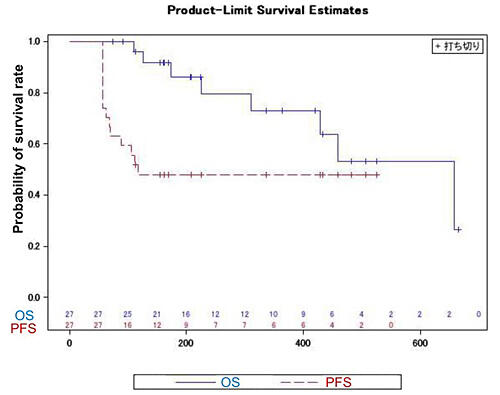
The research group conducted an investigator-initiated clinical trial (phase II) to evaluate the safety and efficacy of Opdivo and PAI-1 inhibitor TM5614, which was provided by Renascience, in 34 patients with advanced melanoma refractory to anti-PD-1 antibodies from September 2021 to March 2023 as a multicenter study at seven medical institutions in Japan (Tohoku University, University of Tsukuba, Tokyo Metropolitan Cancer and Infectious Disease Center Komagome Hospital, Cancer Institute Hospital of Japanese Foundation for Cancer Research, Nagoya City University, Kindai University, and Kumamoto University). A total of 27 patients were included in the PPS analysis at the end of the treatment period. Despite the short duration of combination therapy with Opdivo and TM5614 (8 weeks), the response rate, which was the primary endpoint, was 25.9%, well above the expected response rate of 20% set at the beginning of the study. Regarding safety, which was evaluated as a secondary endpoint, the incidence of serious adverse events for which causal relationship with drugs could not be ruled out was 7.7%, which was far lower than the rates with the standard of care Opdivo + Yervoy (55%-70%) or Yervoy alone (55%-70%). While the drugs were used in combination only for 56 days, progression-free survival, which was an exploratory endpoint, was 174 days (95% CI: 114.4-232.9), indicating that cancer progression did not occur for approximately four months after the completion of combination therapy, and anti-PD-1 antibodies, the main drug in melanoma treatment, began to be effective again. Further improvement in progression-free survival is expected with the combination therapy for a longer period.
Fujimura commented, "About half of Japanese patients with melanoma have acral lentiginous or mucosal melanomas, for which anti-PD-1 antibodies are ineffective. Moreover, gene mutations targeted by molecular-targeted drugs available for use in melanoma treatment are found in only 30% of Japanese patients. Thus, in clinical practice, treatment after non-responsiveness to anti-PD-1 antibodies must be determined at the discretion of the attending physician. Among them, only the Opdivo + Yervoy combination therapy was shown to be effective in clinical trials, but it is not as effective as in initial treatment, and physicians often hesitate to use it because of the high incidence of serious immune-related adverse events."
He continued, "Under these circumstances, the combination therapy in this study had a high response rate in the short term and extended progression-free survival to a median of 6 months after only 8 weeks of treatment, showing more-than-expected outcomes. This indicates that TM5614 reversed the refractoriness to treatment with anti-PD-1 antibodies; thus, the therapeutic effect was sustained for more than 4 months after the completion of the TM5614 combination therapy in half of the patients. Moving forward, to confirm therapeutic efficacy, a phase III study will be conducted to determine the survival benefit of the treatment compared with the placebo group. If the phase III trial results are as hypothesized, we believe that Opdivo + TM5614 will produce complete remissions in >80% of Japanese patients with melanoma requiring systemic therapy for which no treatments are available currently."
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




