A research group led by Researcher Haruki Okamoto, Program-Specific Research Center Junior Associate Professor Sou Nakamura, Professor Koji Eto, and Visiting Researcher Kosuke Fujio (Otsuka Pharmaceutical) of the Department of Clinical Application at the Center for iPS Cell Research and Application at Kyoto University, in collaboration with Satake MultiMix (Toda, Saitama) and the University of Miyazaki, has announced that they successfully designed a bioreactor for generation of clinical-grade platelets up to 45-liter scale. They also identified the causes of problems in the straightforward scaling up of the previously developed bioreactor with a maximum capacity of 8 liters, such as decreased production efficiency and quality. The findings are expected to lead to large-scale culture and accelerate industrialization and were published in the June 17 issue of the international journal Communications Engineering.
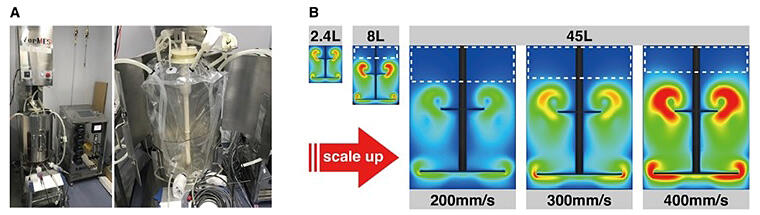
a. Pictures of the developed VerMES bioreactor (left) with zoom on the single-use polyethylene material tank (right).
b. Representative results of turbulent energy for 2.4, 8, 45L scales.
Provided by Kyoto University
While the demand for blood transfusions is expected to continue growing with medical care advancements and an aging population, there are concerns that the demand will not be met through blood donations. Technology to produce platelets in vitro using human induced pluripotent stem cell (iPS) cells is expected to contribute to a stable supply of platelet transfusions independent of blood donors. In response to this need, the research group has been conducting a clinical study (iPLAT1) on autologous transfusion of iPS cell-derived platelets for thrombocytopenia since 2019.
In 2022, they reported the results, including safety confirmation, of transfusion of iPS cell-derived platelets in a patient with alloimmune platelet transfusion refractoriness, in which transfusion causes immunodeficiency due to platelet-specific antigens. In the same study, they successfully established a method for producing megakaryocyte cell lines, which are the sources of platelets, from human iPS cells and an in vitro platelet production technology. They also discovered that turbulence and shear stress are important in the in vivo process of platelet formation from megakaryocytes. Based on this knowledge, they showed that in platelet production culture, vertical blade motion, rather than rotational motion, more efficiently agitated the entire culture tank and induced differentiation into platelets, and developed 0.3-liter, 2.4-liter, and 8-liter vertical motion-type bioreactors in collaboration with Satake MultiMix.
Meanwhile, four 8-liter bioreactors were needed simultaneously to produce the platelets required for transfusion to one patient, and a larger-scale device was needed for practical use to reduce costs and human error. Therefore, they examined the feasibility of scaling up the bioreactor. First, a prototype of a 45-liter plastic film vertical-motion bioreactor was built for commercial use while maintaining the 8-liter bioreactor structure. The 8-liter bioreactor has a blade unit with a shaft penetrating two disk-shaped structures, and the content of the tank is agitated by the vertical movement of the shaft. Platelet production was performed using the prototype 45-liter bioreactor at blade speeds (200, 300, and 400 mm/sec) generating similar turbulent energy levels as the conventional 8-liter bioreactor. Numerical simulations showed that 300 mm/sec would generate a turbulence energy level closest to that with the 2.4-liter or 8-liter bioreactor. When the 45-liter bioreactor was run at three-blade speeds, the production efficiency was markedly decreased to less than half that with the 2.4-liter or 8-liter bioreactors at all speeds. Meanwhile, platelets produced at 300 mm/sec had normal structure and function. The platelets were confirmed to have normal hemostatic and circulation properties in mice, equivalent to those of Japan Red Cross's platelets from blood. The platelets obtained at 200 and 400 mm/sec showed morphological features that normal platelets do not have.
To identify the cause of the reduced production efficiency, computational fluidic dynamics simulations were performed, and the results revealed that the turbulence energy and other stimuli applied to the 2.4- or 8-liter tank were not distributed throughout the 45-liter culture tank. Based on these data, new computational fluid dynamics simulations were performed using a blade unit design with three disk-shaped structures, and the results indicated that the turbulent flow required for production could be generated efficiently. With this blade, platelets of sufficient quality for practical use can be produced efficiently even at speeds as low as 200 mm/sec, at which the likelihood of cell damage is lower. The simulations also indicated that the vertical motion-type bioreactor could be scaled up to a maximum capacity of several tons and that even a rotary-type bioreactor can generate stimuli, including the necessary turbulence energy, by changing the blade structure. Based on the findings of this study, the research group is currently examining appropriate conditions for the rotary type to explore the feasibility of scaling up the development of iPS cell-derived platelets compatible with all types of human leukocyte antigen.
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




