A joint research group led by Associate Professor Kenta Arai and Professor Michio Iwaoka of the Department of Chemistry, School of Science at Tokai University, and Professor Osamu Yoshino and Clinical Assistant Professor Yosuke Ono of the Department of Obstetrics and Gynecology, Faculty of Medicine at the University of Yamanashi, have succeeded for the first time in chemical synthesis of two artificial relaxin analogs (seleno-relaxin analogs) in which one of the three disulfide bonds of human relaxin 2 (relaxin), known as a pregnancy peptide hormone, is replaced by an analogous diselenide bond. They also found that various seleno-relaxin analogs reduced the expression of plasminogen activator inhibitor-1 (PAI-1) mRNA, a tissue fibrosis-related factor implicated in the pathogenesis of endometriosis, by up to 40%. The findings demonstrate that the molecular design of replacing a disulfide bond with a diselenide bond improves the efficiency of relaxin synthesis, suggesting a new strategy for drug discovery for endometriosis. The work was published in the electronic edition of RSC Chemical Biology.
Provided by Tokai University
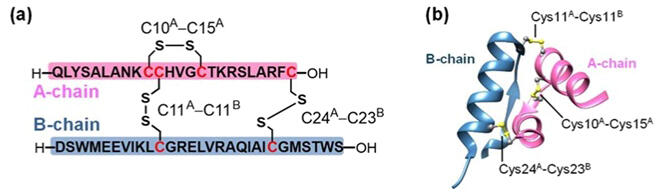
Endometriosis affects approximately 10% of women of childbearing age and induces various conditions that significantly reduce the quality of life of the affected women, such as dysmenorrhea, dyspareunia, and dyschezia. The joint research group reported in 2020 that relaxin may have an inhibitory effect on endometriosis. Relaxin is a protein with a molecular weight of approximately 6000, consisting of two polypeptide chains, A chain (24 amino acid residues) and B chain (29 amino acid residues). The molecular structure is stabilized by two disulfide (S-S) bonds between the A and B chains and one within the A chain. It is technically difficult to selectively form a bond between two different substances. Despite the fact that this protein is very important in the field of drug discovery, its chemical synthesis method has not been established.
In this study, the technology previously used by the Tokai University group for the chemical synthesis of insulin, which is structurally similar to relaxin, was applied to successfully obtain various desired relaxin analogs in a single step by mixing the relaxin A and B chain analogs as source materials in appropriate combinations in the presence of appropriate additives. While wild-type relaxin without diselenide bonds obtained a 47% yield after 48 hours, seleno-relaxin α, in which the disulfide bond exposed on the molecular surface of relaxin was replaced by a diselenide bond, obtained greatly improved yields of the coupling of the A and B chains, with a maximum yield of 73% after 72 hours. Meanwhile, seleno-relaxin β, in which the disulfide bond buried inside the relaxin molecule was replaced by a diselenide bond, was obtained at a maximum yield of 34%; although the maximum yield was slightly lower than that of the wild type, the reaction took <24 hours to complete. In other words, the diselenide bond on the molecular surface controls the yield of coupling, while the intramolecular diselenide bond controls the rate of coupling. Structural analysis of isolated relaxin and various seleno-relaxin analogs suggested that synthetic seleno-relaxin analogs have a structure equivalent to wild-type relaxin.
Previously, the research group has shown that leucine-rich repeat-containing G protein-coupled receptor-7, a receptor for relaxin, is ubiquitously found in endometrial stromal cells derived from endometriosis patients and that relaxin can function as an inhibitory agent for endometriosis. In this study, they investigated the function of synthetic seleno-relaxin analogs on endometrial stromal cells, focusing on their inhibitory effects on the expression of PAI-1. PAI-1 plays an important role in tissue fibrosis, whose elevated concentrations in the intraperitoneal fluid of endometriosis patients have been suggested to contribute to the development of peritoneal lesions. The PAI-1 mRNA expression was significantly reduced in all groups treated with synthetic relaxin (100 nanograms/milliliter). Two seleno-relaxin analogs reduced the PAI-1 mRNA expression more effectively than wild-type relaxin. Seleno-relaxin β at a low concentration (33 nanograms/milliliter) showed the highest efficacy in this measurement. These results suggest the potential of seleno-relaxin analogs as highly effective suppressors for endometriosis in terms of inhibition of PAI-1 production.
Journal Information
Publication: RSC Chemical Biology
Title: Seleno-relaxin analogues: effect of internal and external diselenide bonds on the foldability and a fibrosis-related factor of endometriotic stromal cells
DOI: 10.1039/D4CB00095A
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




