A joint research group led by Associate Professor Yuki Uematsu of the Graduate School of Computer Science and Systems Engineering at Kyushu Institute of Technology and Professor Yasuyuki Kimura of the Graduate School of Science at Kyushu University discovered that within nanobubbles made from just water and air, non-gaseous nanoparticles also exist. Although nanobubbles are already used in industries for cleaning and other uses, advances in fundamental research into their origins can enable progress in a wide range of areas. The research article was published in Physica A: Statistical Mechanics and its Applications on July 6th.
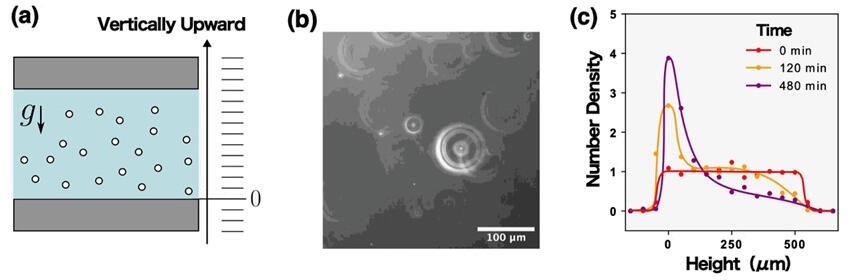
(b). Typical image obtained by dark-field microscopy.
(c). Schematic of the time dependence for the number density of nanobubbles. At the initial stage the nanobubbles distribute uniformly, whereas the late stage the nanobubbles sediment to the lower side.
Provided by Kyushu Institute of Technology
If bubbles in water are large enough, they will rise to the surface and burst. However, nanobubbles with diameters of one micrometer or less are less affected by buoyancy, allowing them to be suspended in water. Theoretically, for a bubble with a diameter of 1 µm, the effect of surface tension increases the internal gas pressure. Furthermore, this causes the gas inside the bubble to dissolve rapidly into water, and the bubble progressively shrinks and disappears within a few milliseconds. This indicates that nanobubbles should not exist stably in water. However, since the 2000s, nanobubbles that stably exist for a long time in water have been observed in a series of experiments. Nevertheless, the question of why nanobubbles are able to exist has been a source of controversy.
Since around 2018, the measurement of the mass of nanobubbles indicates that they are most likely solid or liquid particles rather than bubbles. However, the equipment required to measure their mass was limited, and only two groups in the world had conducted such measurements. On this occasion, the research group could, for the first time, observe how nanobubbles settle due to gravity, through observation in real time using a dark-field microscope developed more than 100 years ago. Because there are no gases heavier than water, the observed nanobubbles were found to be non-gaseous particles. Furthermore, the research group succeeded in calculating the mass density of nanobubbles from their particle number density distributions in a state of sedimentation equilibrium.
As mass measurement was achieved using only a versatile microscope, the method is expected to be easily reproduced by other research groups. Further, the results are expected to lead to future studies focusing on the mass of nanobubbles. Additionally, no such non-gaseous particles have been measured from ultrapure water samples without bubble generation. It was determined that the measured non-gaseous particles of approximately 450 nm diameter are generated during bubble generation.
No evidence was found to deny the existence of nanobubbles with a diameter of less than 100 nm, which were unmeasurable with the research group's experimental system. It has not generally been demonstrated that nanobubbles cannot exist in a stable state for long periods in water. However, the research group showed that at least the nanobubbles generated in their experiments disappeared, potentially contributing to the formation of non-gaseous particles. Micro−nanobubble water has already been put to practical use in various industrial settings owing to its high bioactivity and cleaning effects.
This study clarified that when such micro−nanobubble water is considered on a time scale of several hours, microparticles in water are no longer bubbles and have already transformed into other particles composed of either solids or liquids. Meanwhile, what is currently unknown is whether particles that have remained gaseous after lapse of a few seconds to a few minutes transform into non-gaseous particles after a few hours or whether they have already changed into non-gaseous particles within a span of a few seconds to a few minutes.
The time scale of these bubbles for actual use in cleaning spans a few seconds to a few minutes. Moving forward, therefore, to increase the value of micro−nanobubble water in industrial applications, it is necessary to clarify how bubbles transform into microparticles over short time scales. Additionally, the chemical composition of fine particles is assumed to originate from impurities. However, further research on the source of these impurities and their relationship with the cleaning function of micro−nanobubble water will contribute to further technological developments in micro−nanobubble water.
Journal Information
Publication: Physica A: Statistical Mechanics and its Applications
Title: Nanobubble-assisted formation of non-gaseous nanoparticles in water
DOI: 10.1016/j.physa.2024.129932
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




