A research group led by Associate Professor Fumiaki Ohtake and Graduate students Yuki Mori and Yoshino Akizuki of the Institute for Advanced Life Sciences at Hoshi University, in collaboration with the University of Tokyo, has announced that they discovered for the first time in the world a new principle to enhance the effectiveness of "targeted protein degraders," drugs which remove the intracellular proteins that cause cancer and other diseases. The findings are expected to contribute to developing highly efficient and precise therapeutic agents. The results were published in the international journal Nature Communications on July 2.
Provided by Hoshi University
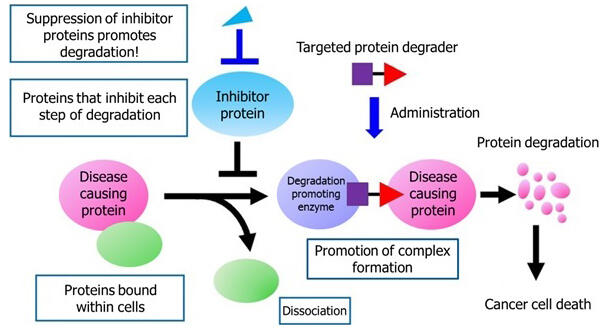
Targeted protein degraders have attracted extensive attention because they can induce the degradation of specific disease-causing proteins to remove them from cells. They are being studied in clinical trials for the treatment of breast cancer, prostate cancer, and other diseases. These degraders utilize the ubiquitin-proteasome system, an intrinsic cellular mechanism in which proteins no longer needed in the cell are labeled with ubiquitin and degraded by proteasomes, which are proteolytic enzymes. Using a hybrid compound in which an agent that binds to a disease-causing protein conjugated with an agent that binds to a degradation-promoting enzyme (ubiquitin ligase), the disease-causing protein is brought into close proximity of the degradation-promoting enzyme, forcibly undergoing ubiquitination and subsequent degradation.
In this study, the group used a degradation inducing agent of BRD4, which is one of the targets of the aforementioned hybrid compound agent, to construct a high-throughput system to measure the induced degradation. With this system, they searched for compounds that promote the degradation of BRD4. BRD4 is a protein that binds to chromatin and recruits a complex necessary for gene expression. Inhibition of this protein prevents gene expression and induces apoptosis in cancer cells. The results showed that pre-administration of known inhibitors improved the efficacy of protein degraders. Analysis of this mechanism revealed that the formation of the target protein complexes necessary for degradation was facilitated because the target protein is inhibited from binding to proteins it normally binds in the cell. In experiments using multiple types of cancer cells, co-administration of these inhibitors promoted BRD4 degradation, altered the global gene expression of the cells, and promoted apoptosis (cell death) of the cancer cells. These results showed for the first time that the degrader effect can be enhanced by creating intracellular conditions that facilitate the degradation of target proteins.
Ohtake said, "If we can improve the effectiveness of protein degraders by clarifying the fundamental mechanism of protein degradation, we expect that this will lead to the discovery of epoch-making drugs that will save patients suffering from cancer and other diseases in the future. This achievement came about from the tireless efforts of Graduate Students Yuki Mori and Yoshino Akizuki."
Journal Information
Publication: Nature Communications
Title: Intrinsic signaling pathways modulate targeted protein degradation
DOI: 10.1038/s41467-024-49519-z
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




