As a method for semiconductor integration, research on three-dimensional large-scale integration (3D-LSI) is progressing, in which the fabrication of through-silicon electrodes in the vertical direction is crucial. Metal-assisted chemical etching (MACE) has attracted attention as a technique for this purpose; however, it requires precious metals, which can cause problems as they remain on silicon. A research team comprising Professor Hiroshi Sakaguchi, Assistant Professor Takahiro Kojima, Doctoral Student Cheng Yingbo, and Assistant Professor Shunpei Nobusue at the Institute of Advanced Energy as well as Associate Professor Kazuhiro Fukami of the Graduate School of Engineering at Kyoto University developed a new carbon wire synthesis method. The research team employed this method to successfully synthesize oxygen-doped graphene nanoribbons (GNRs), which have been difficult to synthesize. The use of this new material as a catalyst overcomes the disadvantages of MACE and is expected to be applied to the 3D-LSI process. The work was published in Nature Communications.
Provided by Kyoto University
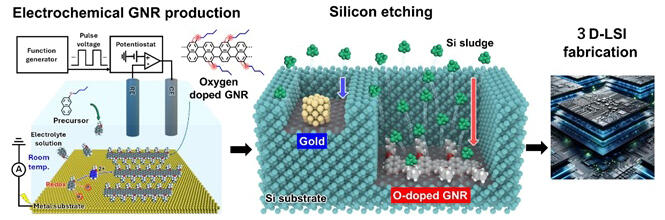
Reactive ion etching is a technique used for etching semiconductors longitudinally. In this method, the substrate is directly bombarded with ions, resulting in significant damage to the substrate, and the method suffers from accuracy problems. MACE is attracting attention in this context. When a catalyst (such as gold) receives electrons from hydrogen peroxide and transfers holes to the silicon, hydrogen fluoride etches it. However, the precious metal in the catalyst remains. Toshiba has abandoned the practical application of this method because of this problem.
In this context, the team wondered, "If we use carbon catalysts instead of metal catalysts, can we solve this problem?" However, fullerenes, carbon nanotubes, and graphene have only one-tenth the catalytic activity of gold. Therefore, they wondered if they could increase catalytic activity by doping graphene nanoribbons with oxygen.
However, using the conventional ultra-high vacuum GNR production method, it is difficult to dope nanoribbons with different elements due to their high cost and temperature. Therefore, the research team developed an electrochemical fabrication method to produce oxygen-doped GNR thin films at room temperature by applying a pulsed voltage to a metal substrate immersed in an electrolytic solution containing raw materials to induce a redox reaction. The mechanism underlying this production technology is that a high voltage is applied to the electric double layer in a one-nanometer-thick space formed at the interface between the metal and electrolyte. The two-electron oxidation reaction, di-cationic polymerization, and ring condensation reaction of raw material molecules occur to synthesize oxygen-doped GNR on metal at room temperature.
By adjusting the duration and number of voltage application pulses, a high-quality GNR thin film with controlled thickness can be formed. The oxygen-doped GNRs developed using this electrochemical manufacturing technique are highly electron-rich and can efficiently oxidize silicon due to their ability to reduce hydrogen peroxide, an oxidant, during silicon etching. They exhibit stronger catalytic activity than gold, a precious metal catalyst. In fact, when MACE was performed on these GNRs, only the areas where the GNRs were deposited on silicon were selectively etched, and the etching rate was the highest among existing catalysts.
Sakaguchi said, "This electrochemical manufacturing technique is based on a plating method in which a metal substrate is dipped in an electrolytic solution to deposit metal, which was published in Science 20 years ago. At that time, it was used in polymers for a completely different application, but this time, by changing the raw material and conditions, we succeeded in synthesizing a substance at room temperature, which was previously possible only at 500℃. The carbon framework of this GNR has a width of two six-membered rings. Nevertheless, by expanding this width or doping with elements other than oxygen, GNRs can be prepared for various applications. We would like to use these results as a starting point for further application research."
Journal Information
Publication: Nature Communications
Title: Electrochemical on-surface synthesis of a strong electron-donating graphene nanoribbon catalyst
DOI: 10.1038/s41467-024-50086-6
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




