A joint research group led by Doctoral Student Takeshi Hara and Professor Hiroshi Sawa at the Graduate School of Engineering at Nagoya University, in collaboration with Professor Toshio Naito at the Graduate School of Science and Engineering at Ehime University, Researcher Yuiga Nakamura at the Japan Synchrotron Radiation Research Institute, and Hokkaido University experimentally observed valence electron distributions in organic molecules for the first time in the world, using X-ray diffraction and scattering experiments at SPring-8, a large synchrotron radiation facility, and first-principles calculations. Through this chemical bonding was successfully visualized, a fundamental problem in chemistry. The proposed method is expected to contribute to the understanding of the nature of chemical bonding, the design of functional materials, and the clarification of reaction mechanisms in the future. This result was published in the electronic edition of Journal of the American Chemical Society.
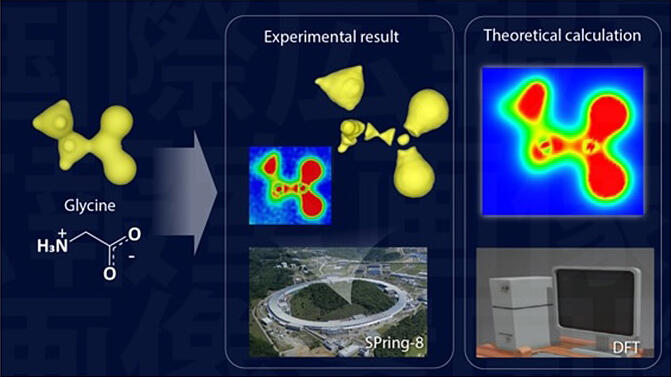
Left: Simple electron model of the glycine molecule.
Center; Top: 3D map, experimentally obtained valence electron density distribution; Bottom: Distant view of the large synchrotron radiation facility SPring-8.
Right: Cross-sectional view of valence electron density obtained through quantum chemical calculations.
The results of the experiment and the quantum chemical calculations show an almost indistinguishable match, providing direct evidence of the ability to extract experimental valence electron density distribution.
Provided by Nagoya University
Valence electrons, those in the outermost shell, are responsible for interactions among atoms. To directly observe the spatial distribution of these electrons, the research group has established the core-difference Fourier synthesis (CDFS) method. Of the electrons in an atom, only the outer-shell electrons (valence electrons) govern the properties of matter, while the inner-shell electrons self-stabilize. To reveal the properties of a material, only the valence electron information needs to be extracted experimentally; however, this is very difficult. As a result, this information has been estimated using theoretical models and spectroscopy.
The research group developed the CDFS method to selectively extract only the valence electron density of atoms constituting a crystal by performing synchrotron X-ray diffraction experiments at SPring-8. The observation of the electronic state of the glycine molecule, an amino acid, using the CDFS method revealed that electrons are not distributed to wrap around the entire molecule in the expected smooth form but are discrete and separated. In particular, electron distributions are broken around carbon atoms.
When carbon forms bonds with surrounding atoms, its electron cloud reconfigures to create hybridized orbitals. In this case, the outermost electrons, the L-shell electrons, have nodes based on their wave nature known as wave functions. In other words, there are nodes in the L-shell distribution due to the wave nature of electrons; hence, even in hybridized orbitals, there are areas where no electrons exist. The distribution state of the interrupted electron cloud obtained in the experiment proves the quantum mechanical wave nature of electrons.
To confirm whether the obtained actual electron cloud captured the true picture or not, state-of-the-art quantum chemical calculations were performed in collaboration with Hokkaido University, and the results were consistent. With a precise understanding of the valence electron density distribution that makes up the glycine molecule, the research group performed similar experiments and calculations on slightly more complex cytidine, extracting information regarding only π electrons in the carbon double bond and clearly observing that the carbon-to-carbon and carbon-to-nitrogen bonds are different.
In the field of chemistry, each researcher's understanding and model of bonding vary considerably. This may be why introductory high school and college chemistry textbooks do not clearly describe bonding. This is because it is difficult to directly compare experimental results with theoretical ones because the necessary information (phase of the wave function) is lost in the experimental results. However, this research has opened up the possibility of directly visualizing the nature of chemical bonding and contributing to the design of functional materials and elucidation of reaction mechanisms. In particular, it is expected to be applied to organic semiconductors and DNA double helices, where π−π interactions affect the material function and structural stability.
Journal Information
Publication: Journal of the American Chemical Society
Title: Unveiling the Nature of Chemical Bonds in Real Space
DOI: 10.1021/jacs.4c05673
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




