Hokkaido University and their collaborators have developed a high-speed spinning technology to produce thin thread-like collagen microfibers that are structurally similar to the collagen fibers that form tendons and ligaments. Bundles of thread-like collagen with hardness and strength about one-half to one-third of healthy human ligaments and are strong enough for clinical application were produced. The materials are likely to be useful as artificial tendons for treating anterior cruciate ligament injuries of the knee, which affect many people, including athletes, and are commonly treated by autografting a tendon from a different part of the body.

Provided by Hokkaido University
Tendons that connect muscles to bones, and ligaments connecting bones to bones, are made of collagen. If thin thread-like collagen with a structure comprising fibers aligned in one direction similar to that found in the human body can be produced on a larger scale, it could be used as a material for artificial tendons. However, the production capacities of conventional technologies were up to a few dozen meters per hour and insufficient to put thin thread-like collagen into practical use.
Chief Researcher Shunji Yunoki (medical engineering) of Nitta Gelatin Inc. (Yao City, Osaka Prefecture), which manufactures and sells gelatin and collagen peptides, served as a professor at the Institute for the Promotion of Business-Regional Collaboration at Hokkaido University, from April 2022 to March 2024. He was involved in developing a spinning technology for high-speed production of thin thread-like collagen.
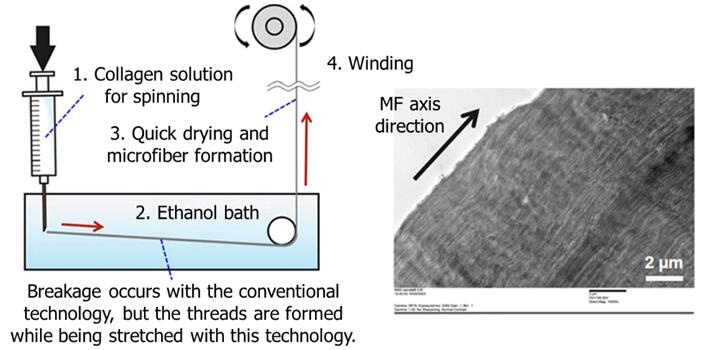
First, based on the long-studied "wet spinning" method, in which an aqueous solution of collagen for spinning is passed through a coagulant and an ethanol bath before the collagen is wound as fiber through a nozzle, the team developed an improved method in which coagulation occurs in the ethanol bath without passing the aqueous collagen solution through the coagulant. This method shortens the spinning process and eliminates the need to remove the chemicals from the coagulant solution.
Next, conventional spinning technology has the problem of breakage during the "stretching" step, in which collagen in the coagulation process is stretched by being pulled in an ethanol bath. The group developed a proprietary aqueous collagen solution of which ingredients were devised to facilitate stretching, and successfully obtained stretched collagen by using a winding speed faster than the extruding speed during the process in which the thread formed by extruding the collagen solution into an ethanol bath was dried and spun to produce microfibers. The stretching step gave an internal structure made up of collagen fibers aligned in one direction.
Provided by Hokkaido University
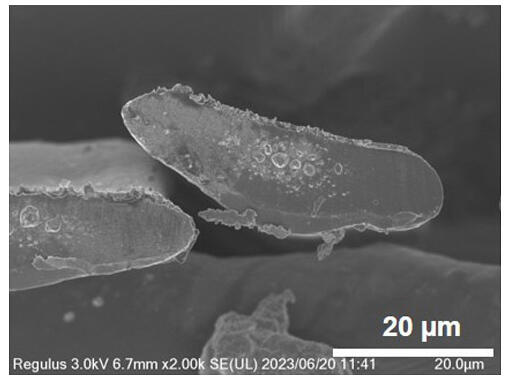
Specifically, stretched collagen was obtained by extruding the aqueous collagen solution for spinning into an ethanol bath at 47 micrometers in diameter and using a winding speed 4.4 times faster than the extruding speed. The spinning speed increased to 200 meters per hour, yielding thread-like collagen with a diameter of 22 micrometers, which was close to 10−20 micrometers of collagen fibers in the body. As a result, continuous production of thread-like collagen has become possible. However, unlike collagen fibers in actual ligaments and tendons, which have perfect-circle cross-sections, the cross-section of the resulting thread-like collagen was oval. This is presumably because of pressure from the rollers during stretching.
Provided by Hokkaido University
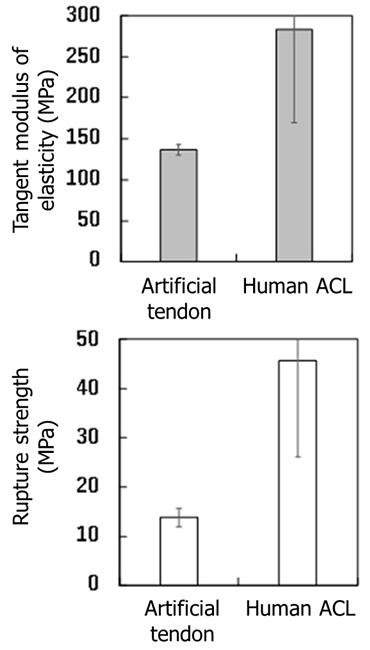
To examine whether thread-like collagen with an oval cross-section could be used as a material for artificial tendons in the medical field, the group evaluated the hardness and strength in terms of elastic modulus and rupture strength, respectively. Tensile tests showed that test artificial tendons made by bundling several hundred thin threads together had elastic modulus and rupture strength that were about one-half and one-third, respectively, of the values of the human anterior cruciate ligament of the knee. The anterior cruciate ligament of the knee was compared to that of a healthy human, and the elastic modulus and rupture strength were weak immediately after grafting a tendon surgically collected from a different location. Thus, the test artificial tendons were considered strong enough for clinical application. The strength can be increased by bundling a larger amount of thread-like collagen to make a thicker bundle.
Provided by Hokkaido University
Moving forward, Yunoki's team plans to develop artificial tendons and ligaments closer to actual tendons and ligaments by, for example, changing the cross-sectional shape of thin thread-like collagen from an oval to a perfect circle and devising new ways of making bundles. The team aims to begin clinical studies in humans within 5−6 years at the earliest and clinical trials in 10 years for practical use.
The study was conducted jointly by Hokkaido University and Nitta Gelatin. The results were published on May 21 in the electronic edition of Biomedical Materials, a journal specializing in biomaterials, and outlined in a press release issued by Hokkaido University on July 4.
Original article was provided by the Science Portal and has been translated by Science Japan.




