A new method for analyzing proteins in the proximity of neurotransmitter receptors within synapses in the live animal brain has been developed. This development was conducted by a team made up of Professor Itaru Hamachi, Junior Associate Professor Tomonori Tamura, and Doctoral Student Mikiko Takato (at the time of the study) of the Graduate School of Engineering at Kyoto University. This method, called the PhoxID method, does not require genetic recombination and thus provides an overview of the protein interaction network in a more natural state. The group revealed age-dependent changes in AMPA receptor-proximal proteins in the mouse brain and identified some previously unknown interactions.
Tamura said, "In principle, the method can be applied to the analysis of proximal proteins of any target protein. The key photosensitizers can be easily made in any biochemistry lab, and I hope many people use the method. We did not apply for a patent." The work was published in Nature Chemical Biology.
Provided by Kyoto University
Clarification of the protein−protein interaction network (interactome) of neurotransmitter receptors is important for understanding complex brain functions, such as memory and learning. However, conventional methods have drawbacks.
APEX is difficult to apply in vivo owing to its toxicity, and BioID/TurboID requires the administration of a biotinylating agent to live mice for 7 consecutive days. In addition, both methods require the expression of fusion proteins via genetic recombination, which raises concerns about their impact on tertiary structures and interactions.
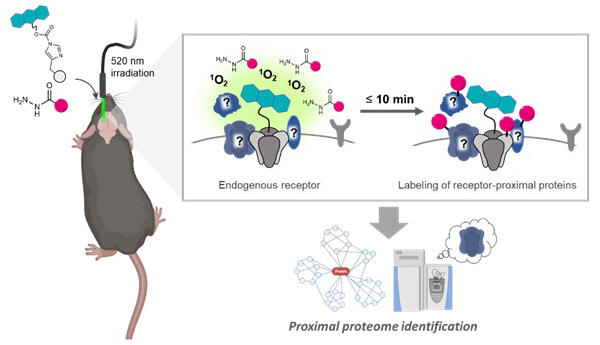
The PhoxID method first uses a proprietary protein chemical modification technique (ligand-directed chemistry) to modify photosensitizers that bind to target receptors. For AMPA receptors, a photosensitizer was prepared by linking the AMPA ligand PFQX to an acylimidazole and attaching monobromofluorescein (MBF) on the opposite side. When introduced into the lateral ventricles, it widely spreads throughout the brain. When the area to be analyzed is irradiated with green light (520 nm) via optical fiber, singlet oxygen is generated, and the proximal proteins are oxidized. The oxidized proteins can be modified with a hydrazide-linked desthiobiotin labeling agent, and the products can be analyzed via mass spectrometry to comprehensively identify the receptor interactome.
Tamura added "Singlet oxygen disappears in a few hundred nanoseconds, during which proteins can diffuse only a few tens of nanometers. So, it can oxidize only proteins in the proximity of the target."
When PhoxID was performed on AMPAR and GABAA receptors in the mouse hippocampus, multiple receptor interactomes, including known interacting proteins, were successfully identified after light exposure for only 1−10 minutes. When the light irradiation site was changed to the cerebellum, known cerebellum-specific AMPAR-interacting proteins were detected. Furthermore, they utilized high temporal resolution to track changes in the AMPAR interactome during postnatal development.
When the PhoxID method was applied to the cerebellum of 8-day-old, 13-day-old, and 5-week-old mice, quantitative mass spectrometry revealed that labeling intensities of many proteins increased with the postnatal development of mice. AMPAR expression increases with brain development, which correlates with the formation of more neural circuits. Meanwhile, labeling intensities of some proteins were highest in 13-day-old mice and decreased markedly in 5-week-old mice. Western blot analysis revealed decreased expression levels of multiple cell adhesion molecules. Immunostaining revealed that the cell adhesion factor NECTIN3 and AMPAR colocalized at 13 days of age, whereas NECTIN3 was expressed separately from AMPAR in cerebellar Purkinje cells at 8 days of age.
The PhoxID method revealed for the first time that NECTIN3 is in proximity to AMPAR in the cerebellum, specifically in juvenile mice. The neuroscientific significance of this phenomenon is unclear, but a more detailed examination would provide new insights.
In the future, PhoxID is expected to be applied to a broader range of receptors, as the method can in principle be used with any proteins and species, provided that affinity ligands and antibodies to the receptor are available. Furthermore, combining this method with cell-level functional analysis based on behavioristics and optogenetics is expected to elucidate the molecular mechanisms of higher brain functions.
Journal Information
Publication: Nature Chemical Biology
Title: Photoproximity labeling of endogenous receptors in the live mouse brain in minutes
DOI: 10.1038/s41589-024-01692-4
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




