A research group made up of Assistant Professor Yoshiyuki Soeda, Graduate Student Riki Koike, Professor Akihiko Takashima, and Assistant Professor Isshin Shiiba of the Department of Life Science, Faculty of Science at Gakushuin University; Assistant Professor Hideaki Yoshimura of the Graduate School of Science at the University of Tokyo; and Professor Hiroko Bannai of the Faculty of Science and Engineering at Waseda University has announced that they have clarified the aggregation process of tau protein with a specific mutation observed in the brains of patients with neurodegenerative diseases collectively known as tauopathies, including Alzheimer's disease. The results are expected to contribute to the understanding of the pathogenesis of these diseases and were published in the international journal Structure on July 20.
Provided by Gakushuin University
Tau protein is present in cells and binds to the cytoskeleton, which stabilizes microtubule structures. Meanwhile, in neurodegenerative diseases such as Alzheimer's disease, tau protein dissociates from microtubules due to abnormal phosphorylation and other factors, transitioning from a water-soluble state to insoluble aggregates.
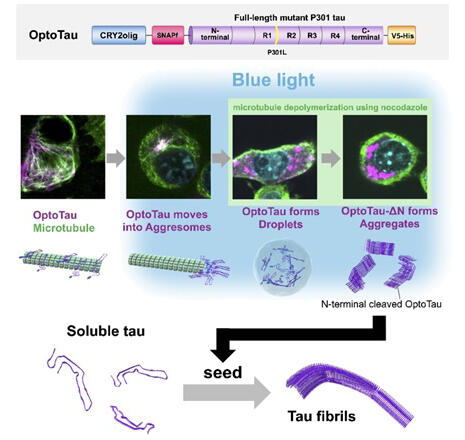
In this study, OptoTau, a tau protein with the aggregation-prone P301L mutation fused with the light-sensitive protein CRY2olig, was used to manipulate tau protein behavior in real time in response to light irradiation. The use of the light-sensitive protein CRY2olig (a mutant of a protein from the plant Arabidopsis thaliana) allows for controlling the movement of tau protein, providing clues to understanding the mechanisms of disease progression. Blue-light irradiation changed OptoTau to a phosphorylated state, as observed in the disease, and promoted the formation of intracellular tau clusters. The clusters were observed to be concentrated around the cell nucleus and sequestered in aggresomes, which constitute a system to process aggregated proteins.
When a microtubule formation inhibitor (nocodazole) was used to destroy aggresomes, OptoTau molecules were scattered in the whole cell during blue-light irradiation, forming membrane-free structures instead of aggresomes. These structures are likely droplets formed by liquid−liquid phase separation (a phenomenon in which two or more liquids form separate phases without mixing), one of the current topics of interest in life sciences. This indicates that the transition of tau protein from a droplet-like structure to a solid mass was observed. N-terminal-deleted OptoTau was also found to form stronger aggregates than OptoTau upon exposure to blue light. This was shown to function as a seed that converts normal tau to tau fibrils. Furthermore, that form of tau had the ability to convert normal tau into abnormal tau aggregates.
Soeda said, "This study was initiated at a reception for the innovative area 'Singularity Biology' through my long-standing friendship with Dr. Hideaki Yoshimura of the University of Tokyo and Dr. Hiroko Bannai of Waseda University. Thanks to my boss, Dr. Akihiko Takashima, and other co-authors of the paper for their support, we obtained clues to the mechanism of tau protein-mediated dementia progression. Based on these results, we will explore inhibitors that could lead to the discovery of tau-targeted drugs for dementia."
Journal Information
Publication: Structure
Title: Intracellular tau fragment droplets serve as seeds for tau fibrils
DOI: 10.1016/j.str.2024.06.018
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




