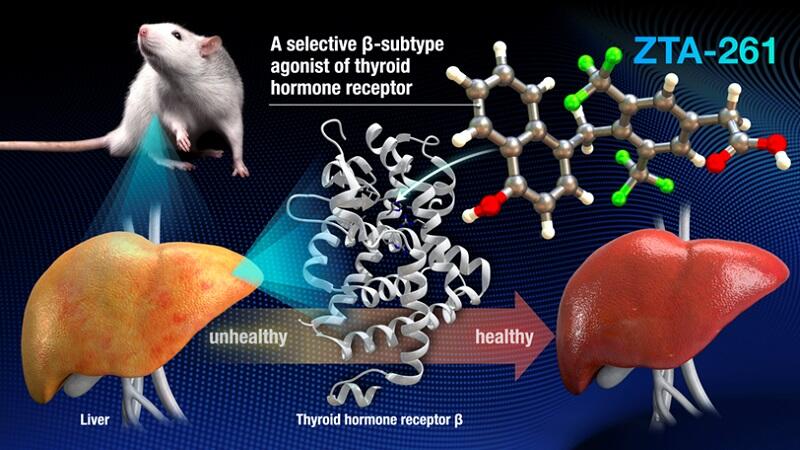
The research group of Designated Associate Professor Masakazu Nambo, Designated Associate Professor Ayato Sato, Visiting Professor Cathleen M. Crudden, Professor Takashi Yoshimura and Associate Professor Taeko Ohkawa-Nishiwaki of the Institute of Transformative Bio-Molecules (WPI-ITbM) at Nagoya University, announced the development of a new thyroid hormone (TH) derivative, ZTA-261, that improves dyslipidemia. The compound was designed based on the structures of existing compounds that selectively bind to THRβ, one of the two TH receptors (THRα and THRβ), in order to suppress side effects mediated by THRα. The compound administered to obese mice reduced blood and liver lipids and was particularly effective in reducing triglycerides. They also confirmed marked reduction of side effects, such as liver dysfunction, compared to existing TH derivatives. The compound is expected to be put to practical use as a new drug for treating dyslipidemia. The results were published in the international journal Communications Medicine on August 6.
Provided by Nagoya University
Approximately 10% of the world's population is classified as obese or overweight, and this number is expected to increase further. Obesity is a major global public health challenge because of its association with the increased risk for hypertension, diabetes, and dyslipidemia. The drugs commonly used to treat dyslipidemia include statins, which inhibit cholesterol biosynthesis in the liver, and ezetimibe, which inhibits the absorption of food-derived cholesterol in the small intestine. However, they are not effective in all patients.
In contrast, thyroid hormone (TH) had the potential of exerting the effect by activating metabolism via THRβ. Meanwhile, a natural TH (T3) has no selectivity for THRα or THRβ. Excessive TH administration can cause serious side effects such as loss of muscle mass, arrhythmia, and reduced bone mineral density via THRα. Due to these effects, therapeutic agents utilizing the properties of thyroid hormones have not been developed.
In this study, the research group investigated the design of compounds that exhibit high selectivity and affinity for THRβ. Molecules were designed based on the requirements previously reported to increase the THRβ selectivity, i.e., increased hydrophobicity and the perpendicular arrangement of the two benzene rings constituting the compound. Three candidate compounds were successfully synthesized. Of these, ZTA-261 had high affinity and selectivity and was examined in detail. The THRβ binding preference (selectivity) of ZTA-261 was compared with that of natural T3 and the existing TH derivative GC-1.
The results showed that ZTA-261 was approximately 100 times more selective than natural T3. GC-1 was approximately 20 times more selective than natural T3. Moreover, mice fed a high-fat diet for 8 weeks to induce obesity received T3, GC-1, ZTA-261, or saline for 3 weeks, and their effects on lipid metabolism and adverse effects on the liver, heart, and bone were examined. ZTA-261, T3, and GC-1, lowered serum and liver triglyceride levels. Furthermore, hepatic, cardiac, and bone toxicities of ZTA-261 were lower than those of T3 and GC-1 and did not differ significantly from those in the saline group, indicating that ZTA-261 may be highly safe.
Ohkawa-Nishiwaki said, "High THRβ selectivity was theoretically assumed to be associated with low side effects through THRα. This was demonstrated by our study, and I think this direction was not wrong. If we were to develop new drugs in the future, we would have to look for ones with higher selectivity and better pharmacokinetics in the body, but there is a limit to what we can do on our own. Moving forward, we hope to conduct extensive collaborative research."
Sato said, "Even if the results are actually published as a paper, it does not mean that the drug will be on store shelves tomorrow. We believe that cooperation with industry will become more important in the future. What we provide are the research results, but we will continue to do our best in research to deliver drugs to more people."
Journal Information
Publication: Communications Medicine
Title: Synthesis and preclinical testing of a selective beta-subtype agonist of thyroid hormone receptor ZTA-261
DOI: 10.1038/s43856-024-00574-z
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




