A research group led by Assistant Professor Mai Sekine and Professor Emeritus Kimiyoshi Ichida of the School of Pharmacy at Tokyo University of Pharmacy and Life Sciences, in collaboration with the University of Tokyo, University of California (USA), and Nippon Medical School, announced that they demonstrated the significance of the purine salvage pathway in the human brain and found a way to amplify its activity. The study provides experimental evidence that the purine salvage pathway is more efficient in ATP synthesis and maintenance than the de novo pathway. Maintaining ATP levels at saturation via the purine salvage pathway is expected to be a promising therapeutic strategy for neurodegenerative diseases and diseases with pathologies of energy depletion and ischemia. The results were published in Journal of Biological Chemistry, an international journal, on July 1.
Epidemiological studies have shown that patients with hyperuricemia and gout have a low risk of developing dementia. Oxidative stress is involved in the pathophysiology of neurodegenerative diseases. The strong antioxidant effect of uric acid is considered a mechanism for reducing risks involved in these conditions. The association between hypouricemia and the increased risk for neurodegenerative diseases was also thought to be attributable to a reduced antioxidant capacity caused by low blood uric acid levels. Meanwhile, the relationship between human brain uric acid levels and neurodegenerative diseases was not well understood.
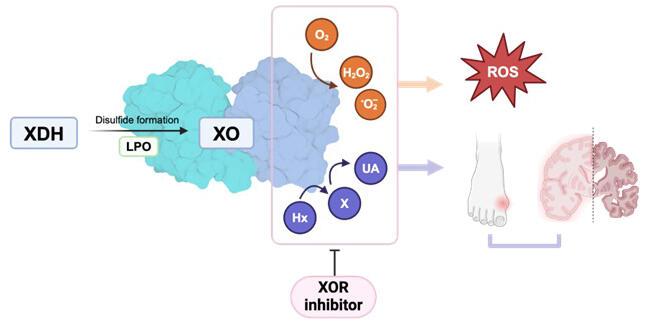
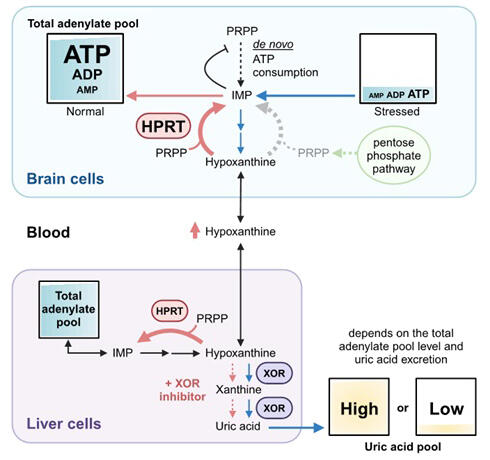
Uric acid is formed through successive reactions from hypoxanthine to xanthine and then from xanthine to uric acid, catalyzed by xanthine oxidoreductase (XOR). In this study, the research group analyzed XOR expression, products, and activity using human brain tissue and iPS cell-derived neurons and found that XOR was not expressed in various brain regions and the uric acid level was as low as 1% of total purines. In contrast, the expression of hypoxanthine phosphoribosyltransferase (HPRT), an enzyme playing a central role in the purine salvage pathway (a pathway to synthesize purine nucleotides using recycled purine bases), was high in the brain, indicating significant hypoxanthine accumulation. The absence of XOR and high HPRT levels in brain cells were significant because the brain requires large amounts of energy to keep hypoxanthine available for the purine salvage pathway.
Provided by Tokyo University of Pharmacy and Life Sciences
ATP is the main intracellular purine, and when purine degradation continues due to sustained stress, it is released out of the cell primarily as hypoxanthine and the total intracellular adenylate level (ATP + ADP + AMP) decreases. If intracellular adenylate loss is not replenished, cellular damage can occur, leading to cell death in extreme cases. Under stress conditions, XOR caused the extracellular release of hypoxanthine as nonreusable uric acid before it can be salvaged, reducing the total intracellular adenylate level. XOR inhibitors, used for treating hyperuricemia and gout, prevent further reduction of the total intracellular adenylate level, thereby exerting cytoprotective effects.
In various organs where XOR is present, these inhibitors increased extracellular hypoxanthine levels by blocking the conversion of hypoxanthine to uric acid. Because hypoxanthine can be transported via the blood−brain barrier, it is thought to be taken up and salvaged by neurons. Since maintaining ATP levels is neuroprotective and the total adenylate pool influences uric acid levels, the relationship between hyperuricemia and neuroprotection observed in previous epidemiological studies may be due to the presence of a total adenylate pool.
Provided by Tokyo University of Pharmacy and Life Sciences
Sekine said, "Alzheimer's disease and other neurodegenerative diseases are serious diseases impacting the nation in various aspects. The gout medications described in this paper are existing drugs that have been used for a long time. Purines and pentoses are also food components. Therefore, they are assumed to be safe, and early clinical trials are desirable, especially for efficacy. Since the mechanism of action is different from that of existing drugs for neurodegenerative diseases, synergistic effects are expected when used in combination."
Journal Information
Publication: Journal of Biological Chemistry
Title: Significance and amplification methods of the purine salvage pathway in human brain cells
DOI: 10.1016/j.jbc.2024.107524
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




