On July 30, a research group led by Professor Hiroshi Kawamoto, Associate Professor Eihachiro Kawase, and Associate Professor Akiko Makino of the Institute for Life and Medical Sciences of Kyoto University, in collaboration with Fujita Health University, Osaka University, and the National Center for Child Health and Development (NCCHD), announced that they successfully developed a pluripotent stem cell (ES cell)-derived killer T-cell preparation for the treatment of COVID-19. Kyoto University led the patent application. Their immediate goal is to conduct clinical trials at Fujita Health University in patients who are refractory to COVID-19 therapy as a result of cancer treatment, with the aim of commercialization in about 5 years.
Provided by Hiroshi Kawamoto, Kyoto University
No magic bullets against COVID-19 are available yet. COVID-19 remains life-threatening, especially for older patients, people with underlying diseases, and immunocompromised patients, such as those undergoing cancer treatment. Antibody drugs have been developed, but they are only effective in the early stages of infection and less effective against variants.
Killer T cells, an immune-cell type, eliminate virus-infected and cancer cells by recognizing and attacking them based on the antigens they present. This function is used in a cancer treatment method where patients' own T cells are activated and amplified before being reintroduced into the body as well as in CAR-T cell therapy, which involves introducing a chimeric antigen receptor (CAR) into patients' peripheral blood T cells. These therapies are effective for certain cancers and have been approved in many countries, including Japan. Meanwhile, these T-cell therapies have issues related to the use of autologous cells, such as the several weeks required to culture the cells, variability in cell quality, and high costs.
To overcome these challenges, Kawamoto and his colleagues have been developing iPS cell- and ES-cell-based technologies for producing killer T-cell preparations since 2004. First, to clone T-cell receptors (TCRs) that can recognize severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-infected cells and match the HLA types commonly found in Japanese people, they performed HLA typing on healthy, vaccinated individuals and selected donors with HLA-A2402 or HLA-A0201, which are common among Japanese individuals. Peripheral blood mononuclear cells were collected from these donors, and T cells specifically recognizing the SARS-CoV-2 S protein, which account for a few percent of the cells, were isolated. The TCR gene sequences were then decoded. They succeeded in obtaining three different TCRs corresponding to HLA-A2402 and two different TCRs corresponding to HLA-A0201.
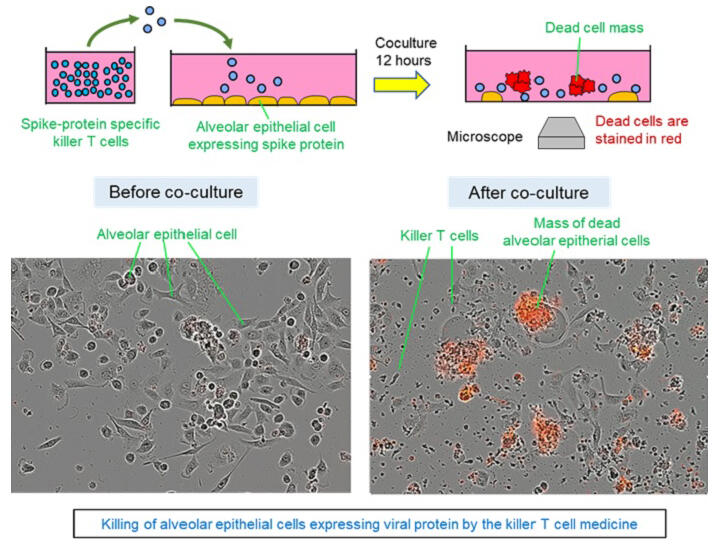
To avoid rejection, regenerative killer T cells were induced from HLA-deficient ES cells prepared by genome editing, and the TCR gene was then introduced using a retrovirus. To verify the function of the regenerative killer T cells prepared, they were added to an alveolar epithelial cell line forcibly expressing the SARS-CoV-2 S protein at a 1:1 ratio. It was confirmed that the regenerative killer T cells began killing the abovementioned alveolar epithelial cells 2-3 hours after addition, killing 90% of these cells within approximately about 12 hours. The prepared regenerative killer T cells are not attacked by the patient's own killer T cells and can exert a therapeutic effect because they lack HLA. Although HLA-deficient killer T cells may eventually be attacked by NK cells, there is a grace period of a few weeks, which is sufficient to achieve therapeutic efficacy. Similar regenerative killer T cells can also be prepared from iPS cells; however, production costs are lower when using ES cells, as the ES cell patent is less restrictive, and the necessary patents were obtained by the research group.
The research group plans to develop cell formulations that can be legally tested in clinical trials at a GMP-grade cell formulation facility and to confirm their safety and efficacy using cultured cells and immunodeficient mice. Since the TCRs of the cells prepared in this project are restricted to HLA-A2402 or HLA-A0201, the research group plans to generate killer T cells with other HLA types. Approximately 60% of Japanese individuals have HLA-A2402, and clinical trials use the preparation with this HLA type. T-cell preparations with approximately 10 of the most frequently found HLA types are expected to enable treatment for at least 90% of Japanese patients. As this method can be applied to other viruses and the cells can be cryopreserved, it is possible to prepare killer T cells ahead of pandemics.
Kawamoto said, "What I want to emphasize is that this treatment method can be applied not only to SARS-CoV-2 but also to other viruses. For example, hundreds of hematopoietic stem cell transplant recipients have died from cytomegalovirus and EB virus due to viral reactivation after transplantation. Parallel research to save these patients is already being conducted at Fujita Health University."
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




