A research group led by Assistant Professor Takayuki Nagae, Associate Professor Hiroshi Aoyama, and Professor Masaki Mishima from the School of Pharmacy at Tokyo University of Pharmacy and Life Sciences, in collaboration with Toyohashi University of Technology, the Faculty of Science and Engineering at Saga University, the Graduate School of Natural Science and Technology at Kanazawa University, the Institute for Protein Research at Osaka University, Jichi Medical University School of Medicine, and the Graduate School of Science at Tokyo Metropolitan University, has announced the analysis of the structure and details of the green light-absorbing state (Pg) of cyanobacteriochrome RcaE, a photoreceptor protein in cyanobacteria. They have also clarified the mechanism by which this photoswitch regulates photosynthesis in cyanobacteria. This was achieved by an analysis that combined four methods: X-ray crystallography, chemical synthesis of bilin chromophores, NMR measurements and quantum chemical calculations. The findings are expected to contribute to further clarifying the environmental response mechanism of photosynthesis. The results were published in the international journal Science Advances on June 12.
Provided by Tokyo University of Pharmacy and Life Sciences
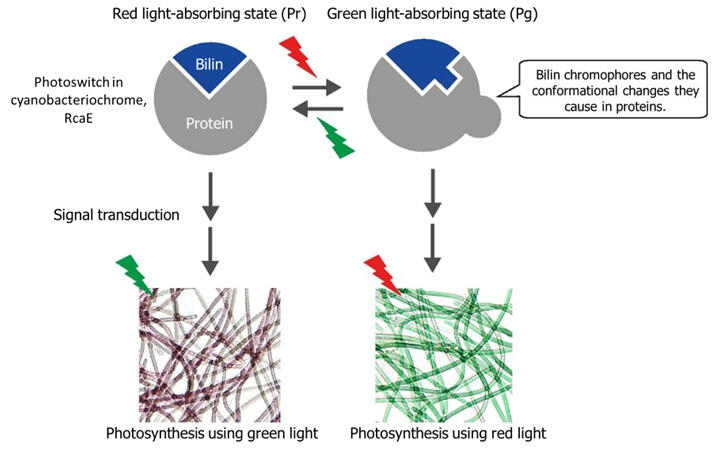
Photosynthetic cyanobacteria alter their photosynthetic light absorption in response to different light to ensure optimal photosynthesis in diverse environments. For example, some cyanobacteria can use red light or green light for photosynthesis according to the environment. Recently, cyanobacteria were shown to possess cyanobacteriochromes, members of the superfamily of photoreceptor proteins that bind a bilin chromophore (light-receiving pigment) and can sense various types of lights. In particular, cyanobacteriochrome RcaE, which senses green and red light, has been shown to act as a photoswitch that regulates the shape of the photosynthetic antenna protein and the color of light it absorbs.
Previously, the research group succeeded in clarifying the structure of the red light-absorbing state (Pr) of the cyanobacteriochrome RcaE from a cyanobacterium capable of photosynthesis using red and green light. However, the structure of the green light-absorbing state (Pg) remained unknown, and structural changes between the two states were not understood. In this study, they focused on RcaE. This photosensor switches the wavelength of light to absorb by changing its own structure in response to the light received in order for cyanobacteria to distinguish between red and green light.
RcaE is composed of a bilin chromophore, which is a light-receiving pigment, and a protein surrounding it. The analysis of the bilin chromophore's structure revealed that in the green light-absorbing state, hydrogen atoms (protons) at specific sites within the hydrophobic environment are removed from the bilin chromophore. This alteration in bonding state shifts the wavelength of absorbed light significantly toward the shorter wavelength side, transitioning from a red-dominant to a green-dominant absorption spectrum.
The group demonstrated the existence of a novel mechanism, in which the absorption wavelength is controlled by switching the chemical property around the bilin chromophore between the hydrophilic and hydrophobic characters. The absorption wavelength of the photoswitch could potentially be significantly modified if it becomes feasible to control the protonation state of the bilin chromophore.
Mishima said, "This protein is characterized by its ability to stably maintain distinct three-dimensional structures in both the green and red light-absorbing states without readily undergoing relaxation (returning to the original state). Therefore, the protein is expected to find application as a novel photo-responsive switch. The protein also has an interesting feature that the maximum absorption wavelength is controlled by protonation of the chromophore. To clarify the deprotonation sites, we directly measured 15N (nitrogen) NMR signals, which have very low measurement sensitivity in protein samples, and succeeded in identifying the sites."
Journal Information
Publication: Science Advances
Title: Green/red light-sensing mechanism in the chromatic acclimation photosensor
DOI: 10.1126/sciadv.adn8386
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




