A research group led by Professor Hitoshi Okazawa, Graduate Student Yuki Yoshioka (currently Specially Appointed Assistant Professor), Graduate Student Yong Huang, Junior Associate Professor Hikari Tanaka, Adjunct Lecturer Kyota Fujita (Project Associate Professor at Kanazawa University), and Specially Appointed Associate Professor Hidenori Homma of the Medical Research Institute at Tokyo Medical and Dental University (TMDU, Institute of Science Tokyo as of October 1st), in collaboration with Professor Toshio Ando of Kanazawa University, demonstrated that functional impairment of PQBP3, a molecule discovered by Okazawa and his colleagues, can explain the link between senescence and neurodegenerative disease pathology at the molecular level. Okazawa commented, "Since functional impairment of PQBP3 can occur in both cellular senescence and neurodegeneration in neurons, targeting PQBP3 can improve both conditions. However, it has also been reported as a risk factor for cancer in dividing cells other than neurons. The development of drugs to prevent senescence and neurodegeneration by activating PQBP3 requires technical innovations, such as the use of a highly neuron-selective regulatory mechanism of expression." The study was published in The EMBO Journal.
Provided by Institute of Science Tokyo
Senescence is the greatest risk factor for neurodegenerative diseases, and the link between them has attracted a great amount of attention. Although therapies are being developed to remove senescent cells in skin and other sites of the body, they cannot be applied to neurodegenerative diseases because neurons do not regenerate after removal. Thus, the evaluating the relationship between senescence and neurodegenerative diseases is important, but the molecular basis of this relationship remains poorly understood.
More than 20 years ago, Okazawa's research group identified several common proteins, including PQBP1, PQBP3, PQBP5, and VCP, that bind to proteins responsible for polyglutamine diseases, serving as a starting point for cytotoxicity. Among these proteins, only PQBP3's function remained unknown. In this study, the research group showed that nuclear PQBP3 is reduced in cellular senescence, binds to disease proteins and is reduced in the cell nucleus in neurodegenerative disease pathologies, and maintains nuclear membrane stability. The research group conclusively demonstrated that cellular senescence and degenerative disease pathologies in neurons have decreased PQBP3 function as a common feature.
Okazawa stated, "Many graduate students have been engaged in this project and produced many data. After carefully reviewing their data, I noticed a correlation between the phenomena occurring in PQBP3 in the nucleolus and the intracellular distribution of DNA, and this is how this study started. From there, we worked hard on the analysis and finally got to see the overall picture."
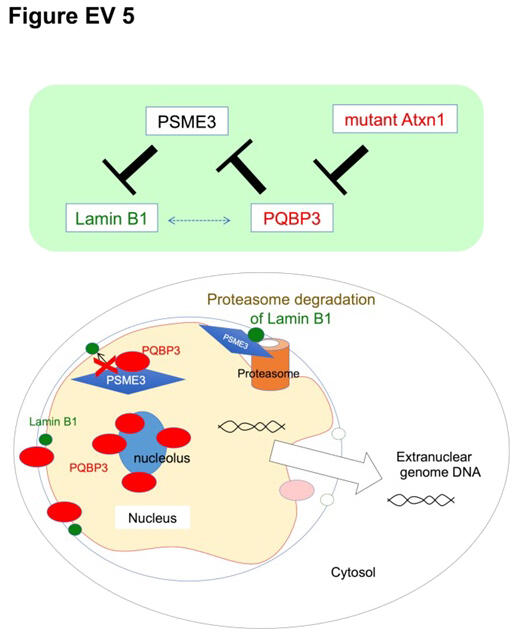
Specifically, the following phenomena occur. Under normal conditions, PQBP3 stored in the nucleolus is translocated in part to the nuclear membrane, where it inhibits PSME3-mediated degradation of the nuclear lamina protein Lamin B1. In senescent cells, PQBP3 is translocated to the cytoplasm through mTOR signaling activation, leading to its deficiency in the nucleus and nuclear lamina. In polyglutamine diseases, such as spinocerebellar ataxia type 1 (SCA1), PQBP3 is also scarce in the nucleus and nuclear lamina because PQBP3 is attracted to inclusion bodies, where the disease protein accumulates and aggregates, causing PQBP3 to be similarly translocated to the cytoplasm.
Okazawa said, "All PQBPs are intrinsically denatured proteins. The liquid−liquid phase separation (LLPS) phenomenon between intrinsically denatured proteins may play a role in neurodegenerative diseases. Currently, LLPS can be observed at the living state only in mouse brains; however, if observation of LLPS in living human brains from outside the body becomes possible in the future, we may be able to detect signs of neurodegenerative diseases."
Journal Information
Publication: The EMBO Journal
Title: PQBP3 prevents senescence by suppressing PSME3-mediated proteasomal Lamin B1 degradation
DOI: 10.1038/s44318-024-00192-4
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




