A joint research group comprising Associate Professor Kazuki Miyata and Professor Takeshi Fukuma of the NanoLife Science Institute (NanoLSI) at Kanazawa University, and Professor Adam Foster (NanoLSI Overseas Senior Researcher) of the Department of Applied Physics at Aalto University, Finland, developed a high-speed three-dimensional scanning force microscope (high-speed 3D-SFM) that can perform 3D observations of solid-liquid interface structures with a subnanoscale resolution at speeds more than 10 times that of conventional techniques. Using this technique, they succeeded in observing the water structure at the solid-liquid interface at a subnanoscale, which changes with the calcite (CaCO3) surface that dissolves in water. The result was published in Nano Letters.
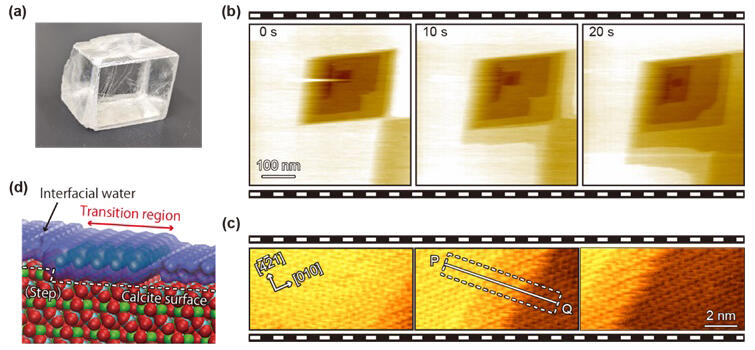
(b) High-speed FM-AFM observation of calcite in water. Calcite dissolves in such a way that parallelogram-shaped holes (pits) are formed on the surface and the steps on the four sides spread out.
(c) High-speed FM-AFM image taken by zooming in on the vicinity of the steps. The moving steps are captured as well as the atomic-scale structure. A transition region formed as an intermediate state in the dissolution process is observed in the vicinity of the step.
(d) A model of the step vicinity based on the results of the high-speed FM-AFM observations.
© 2024 Miyata et al. Published by American Chemical Society
A space that serves as the boundary between solid and liquid (solid-liquid interface) exists throughout the natural world. Water at the interface plays an important role in various phenomena in biology, materials science, earth science, and others. For example, in earth sciences, it is known to be involved in the crystal growth and dissolution of minerals.
To understand the mechanism of these phenomena at the atomic and molecular scales, there is a need to clarify the behavior of water at the interface. However, until now, the mechanisms have not been well understood due to the lack of measurement techniques that allow direct observation. Atomic force microscopy (AFM) is a measurement technique that can solve this problem.
The research group has been working on improving the functionality of such microscopes. For example, they increased the speed of a frequency-modulation AFM (FM-AFM), which enables observations in liquid with the atomic-level resolution, allowing measurements at one image per second. Consequently, they succeeded in observing the dissolution of calcite in water and directly monitoring the atomic-level structural changes in the vicinity of a monomolecular step. Furthermore, they were the first to discover that a transition region with a width of several nanometers forms along this step as an intermediate state of dissolution. However, the mechanism underlying the stable formation of this transition region remains unclear. This is because a high-speed FM-AFM cannot facilitate 3D measurements. The research group also developed a 3D-SFM that enables the 3D observation of solid-liquid interfaces.
They succeeded in visualizing water structures on various materials such as mineral crystals, biomolecules, and polymeric materials at the subnanoscale. However, due to the slow measurement speed of the conventional 3D-SFM, it was not possible to observe the behavior of water, which changes with the surface structure in response to phenomena at the solid-liquid interface.
In this study, the researchers focused on the high-speed FM-AFM they have fabricated. Based on this, they developed a technology to accelerate 3D-SFM measurements. As a result, the measurement speed was improved by more than 10 times that of conventional systems, successfully achieving a maximum acquisition speed of 1.6 seconds per 3D image.
Using this newly developed high-speed 3D-SFM, they observed calcite in water and succeeded in visualizing water in the transition region near the step edge in 3D, which continued to move in response to calcite dissolution. They also found that the water structure in this transition region was completely different from that on the flat areas (terraces) of the calcite surface. Furthermore, based on a combination of simulations, measurements, and previous research results, the following transition region formation model was proposed.
Water structures on terraces before dissolution and on calcium hydroxide, which appeared as an intermediate state during dissolution, formed a hydrogen-bonded network. This network acted as a lid, inhibiting the desorption of calcium hydroxide from the calcite surface and resulting in the formation of a transition region. The stability of the network decreased with increasing distance from the step edge. The distance at which calcium hydroxide desorption can no longer be inhibited determined the width of the transition region.
Thus, the developed high-speed 3D-SFM enables the observation of the structure and behavior of interfacial water, which affects solid-liquid interface phenomena such as crystal growth and dissolution, self-organization, and metal corrosion and catalytic reactions not only in calcite but also in various minerals, organic molecules, and biomolecules. It is expected to contribute to the advancement of research in a wide range of academic and industrial fields.
Journal Information
Publication: Nano Letters
Title: High-Speed Three-Dimensional Scanning Force Microscopy Visualization of Subnanoscale Hydration Structures on Dissolving Calcite Step Edges
DOI: 10.1021/acs.nanolett.4c02368
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




