A research group led by Graduate Students Kei Saito and Kensei Umeda, Assistant Professor Kotaro Fujii, and Professor Masatomo Yashima of the Department of Chemistry, School of Science at Tokyo Institute of Technology (Currently the Institute of Science Tokyo), announced the discovery of a new material, BaSc0.8W0.2O2.8, which exhibits the highest proton (H+: hydrogen ion) conductivity reported ever in the mid-low temperature range, was prepared using a new material design strategy. They clarified this factor based on a crystal structure analysis and theoretical calculations using neutron diffraction data measured in collaboration with Professor Kazuhiro Mori of the Institute of Materials Structure Science at the High Energy Accelerator Research Organization (KEK). The development of proton ceramic fuel cells (PCFC) with high performance at medium to low temperatures is expected. The results were published in the May 3 issue of the Journal of Materials Chemistry A.
Provided by the Institute of Science Tokyo
Proton conductors are materials that conduct H+ and are gaining attention as clean energy materials that can be applied to various electrochemical devices such as PCFCs, hydrogen pumps, and hydrogen sensors. In general, H+ exhibits higher electrical conductivity than oxide ions (O2−) at low temperatures because of its smaller ionic radius, oxidation number, and lower energy barrier for diffusion. Therefore, PCFCs are expected to function at lower operating temperatures than conventional solid oxide fuel cells (SOFCs) that use proton conductors as electrolytes.
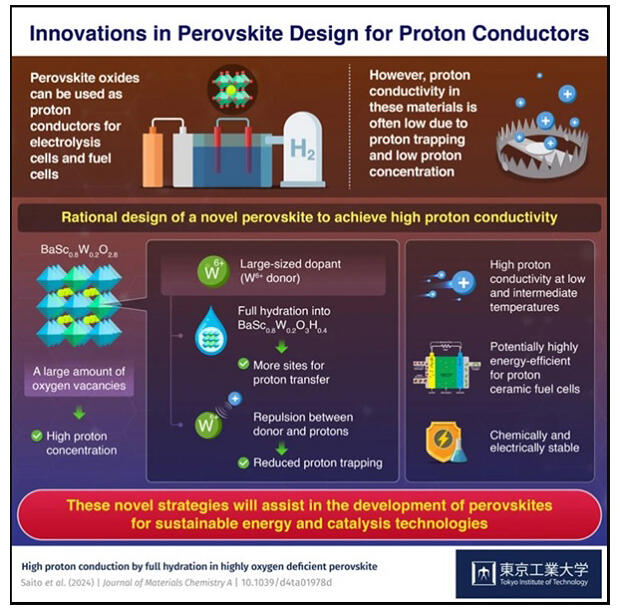
In general, proton conduction occurs when the O in H2O fills oxygen vacancies, and the generated protons diffuse through the material. Therefore, to achieve high proton conductivity, it is necessary to introduce oxygen vacancies in the crystal structure via acceptor doping. However, in this method, protons are trapped by acceptors with a negative effective charge, resulting in a lower proton diffusion coefficient at medium to low temperatures (50℃-500℃). Proton conductors fabricated using this method have a low number of oxygen vacancies and low proton concentrations.
The research group has previously reported that Ba2LuAlO5 and other materials with intrinsic oxygen vacancies exhibit high proton conductivity at medium to low temperatures without doping. By reducing proton trapping via doping of such oxygen vacancies with a donor, the group has discovered a proton conductor BaSc0.8W0.2O2.8, which exhibits the world's highest proton conductivity then at medium to low temperatures.
Meanwhile, the fact that the prepared material did not have 100% water uptake rate was an issue. Therefore, in this study, the research group added W6+, a potential donor, to BaScO2.5, a material with irregularly arranged intrinsic oxygen vacancies, which has been reported to exhibit high proton conductivity when a Mo6+ donor was added. Thus, a new material BaSc0.8W0.2O2.8 was successfully synthesized.
This new material has more oxygen vacancies than the previously reported proton conductor, and its complete hydration (water uptake ratio: 1.0) contributed to its high proton concentration. The activation energy calculated from the diffusion coefficient was lower than that of conventional acceptor-doped proton conductors. Furthermore, the material's high diffusion coefficient owing to its low activation energy is one of the factors contributing to its high proton conductivity at medium and low temperatures. The advantages of PCFCs include the elimination of the need for expensive platinum and heat-resistant materials and ability to operate at high speeds.
Yashima said, "To show high proton conductivity, it is necessary to measure electrical conductivity. Water uptake can cause a sample to crack due to chemical expansion. The fabrication of sintered samples for electrical conductivity measurement and the performance of reliable measurements were challenging. It is expected that research and development of materials with intrinsic oxygen vacancies will gain momentum in the future. We also believe that electrochemical devices will be developed in the future using the newly discovered BaSc0.8W0.2O2.8."
Journal Information
Publication: Journal of Materials Chemistry A
Title: High proton conduction by full hydration in highly oxygen deficient perovskite
DOI: 10.1039/D4TA01978D
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




