A research group led by Professor Schuichi Koizumi and Professor Eiji Shigetomi of the Interdisciplinary Graduate School of Medicine at the University of Yamanashi, in collaboration with Kyushu University, Keio University, and the University of Tokyo, announced discovery of IGFBP2, an insulin-like growth factor-binding protein, as a glial substance that causes brain hyperexcitation. The discovery was made by analyzing mouse models overexpressing the P2Y1 receptor, which is highly expressed in astrocytes in a variety of diseases, including epilepsy, cerebral infarction, and Alzheimer's disease. These results are expected to contribute to the elucidation of the pathophysiology of related diseases. The study was published in Nature Communications on August 8.
Provided by the University of Yamanashi
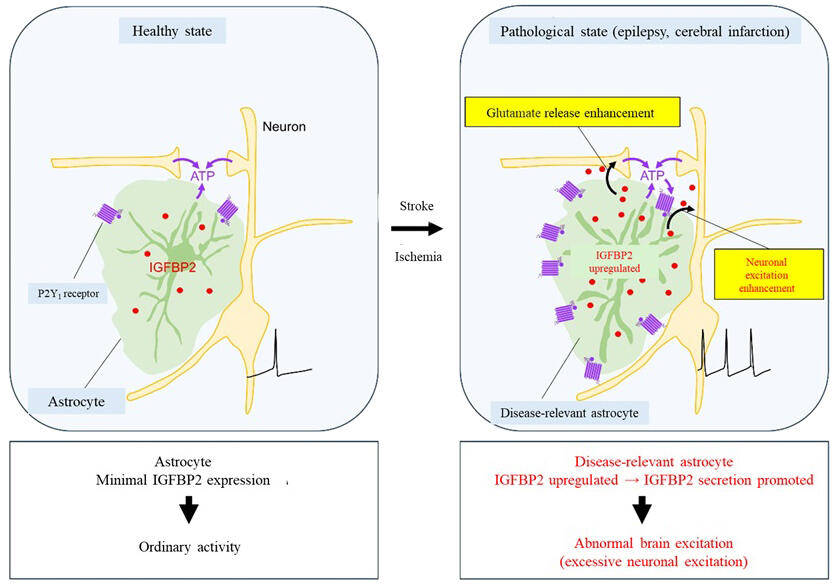
The brain is made up of neurons and non-neuronal cells, with glial cells constituting the majority of non-neuronal cells. Glial cells respond quickly to abnormal changes occurring inside and outside the brain and undergo major property changes. Recent studies have shown that glial cell changes are closely associated with various brain diseases. Astrocytes, a type of glial cells, quickly change their morphology, function, and gene expression in response to injury or inflammation and turn into disease-associated astrocytes, which are completely different astrocytes.
Disease-associated astrocytes are known to cause hyperexcitation of neurons, which leads to neuronal injury and cell death. Disease-associated astrocytes in animal models of various pathological conditions, such as epilepsy, cerebral infarction, and Alzheimer's disease, are commonly characterized by high P2Y1 receptor expression. Meanwhile, the mechanism of how disease-associated astrocytes cause these brain diseases is not understood yet.
In this study, the research group used genetic recombination technology to induce disease-associated astrocytes overexpressing the P2Y1 receptor. The results demonstrated that disease-associated astrocytes cause brain hyperexcitation. Increased action potentials produced by hippocampal neurons, abnormal hippocampal spikes, and susceptibility to drug-induced epilepsy were observed.
To investigate the cause of these increases, the activities of neurons and astrocytes were examined via Ca2+ imaging. The results showed increases in signaling from neurons to disease-associated astrocytes, signaling from disease-associated astrocytes to neurons by certain substances, and signaling between neurons. They also found that these increases resulted from increased release of the excitatory neurotransmitter glutamic acid. Furthermore, through a comprehensive analysis of genes expressed in disease-associated astrocytes, "IGFBP2" was noted to cause brain hyperexcitation.
The administration of IGFBP2 inhibitors or astrocyte-specific gene deletions eliminated brain hyperexcitation. The group also confirmed that "IGFBP2" increased in astrocytes in various brain diseases, such as epilepsy and cerebral infarction.
Koizumi said, "IGFBP2, which we discovered in this study, is a substance produced and released by 'astrocytes,' a type of glial cells, during brain diseases and induces hyperexcitation and death of neuronal cells. Interestingly, IGFBP2 is commonly produced by astrocytes in different diseases. Thus, IGFBP2 is predicted to be a common etiologic agent in various brain diseases. Moving forward, I hope that the regulation of astrocytes and IGFBP2 will lead to the elucidation of common etiologies, the development of therapeutic strategies, and other achievements."
Journal Information
Publication: Nature Communications
Title: Disease-relevant upregulation of P2Y1 receptor in astrocytes enhances neuronal excitability via IGFBP2
DOI: 10.1038/s41467-024-50190-7
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




