A Japanese medical team said Friday it has applied to obtain approval to conduct a clinical study to temporarily transplant pig kidneys into unborn children with severe kidney disease.
Animal-to-human transplants have never been conducted in Japan, and the team aims to be the first to do so in 2026.
The team, which includes the Jikei University School of Medicine and the National Center for Child Development, has submitted the plan of the clinical study for approval with the university's special committee comprised of experts in regenerative medicine, bioethics and lawyers.
The team will subsequently need to apply for approval from other organizations, such as the state-designated committee, to go ahead with the procedure.
Under the plan, two unborn children diagnosed with potter sequence, a disease in which the child cannot produce enough urine, will be transplanted with a kidney, measuring about 2 millimeters, taken from a fetal pig.
The operation will be conducted four weeks before the due date. The pig's kidney will be injected by a hypodermic shot under the skin of the unborn baby's back, enabling the fetus to produce urine right after birth, the team says.
After the baby is born, a tube will be inserted into the baby's back, to allow it to discharge urine.
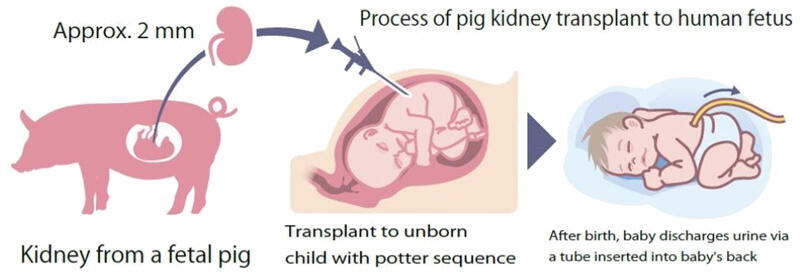
It will be a temporary measure, and the pig's kidney will be removed a few weeks after birth, once the child has grown enough to qualify for dialysis treatment.
Animal-to-human transplants typically harbor higher risks of transplant rejection, where the recipient's immune system rejects the transplanted tissue. However, risks are lower using tissue from a fetal pig, and the amount of immune-suppressing drugs needed can be very small, says the team.
Pig-to-human transplants have been conducted in the United States, and are expected to help solve the organ donor shortage since the organs are similar in size to humans.
Ethical issues remain, and since this study would involve an unborn baby, the university's special committee will review the safety and ethics of the plan.




