A research group led by Specially Appointed Researcher Shunsuke Mori and Professor Hisashi Arase of the Immunology Frontier Research Center at Osaka University discovered that autoimmune diseases are caused by the presentation of abnormal self-antigens (neoself) and the subsequent immune responses to them. This occurs due to dysfunction in the major histocompatibility complex (MHC), which is responsible for presenting antigens to T cells, the command center of immune responses. This is an achievement that changes the basic principle of immunology. Arase commented on the result, "The etiology of most autoimmune diseases may be explained by neoself. The development of new treatments targeting the etiology can be expected."
Provided by Osaka University
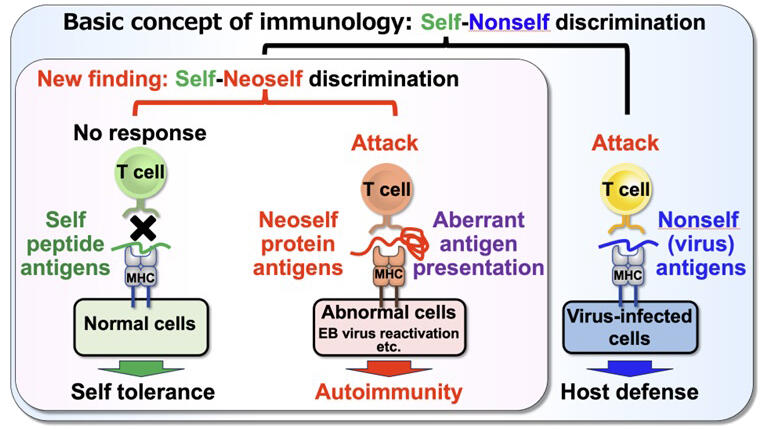
A certain MHC genotype is the strongest genetic risk factor for most autoimmune diseases. T cells recognize antigens such as viruses presented by MHC. However, they do not respond to self-antigens because they become tolerant (nonresponsive) to self-antigens presented by MHC during differentiation and maturation. This is called the self-nonself discrimination by T cells and is a fundamental concept in immunology. However, this traditional basic concept alone could not explain why immune responses to specific self-antigens are elicited in autoimmune diseases and why an MHC genotype is the strongest genetic risk factor.
The research group discovered an abnormal cellular condition in which self-antigens were presented on MHC via an intracellular pathway different from the one previously thought. They named these antigens presented on the MHC of these abnormal cells "neoself."
Arase said, "In the course of a completely different study, I accidentally discovered in 2013 a mechanism by which MHC transports abnormal molecules to outside of the cell."
In this study, the research group analyzed the reactivity of T cells to the neoself antigens and discovered the antigens presenting on MHC due to dysfunction are the causative molecules of systemic autoimmune diseases. To investigate their effects, the research group established mice in which neoself antigen presentation was induced via drug administration.
They found that induction of neoself antigen presentation in mature mice resulted in the development of an autoimmune disease similar to systemic lupus erythematosus (SLE), a systemic autoimmune disease, in which neoself-reactive T cells proliferate markedly and various autoantibodies are produced. Furthermore, T cells recognizing neoself antigens were found to have pathogenicity to induce autoantibody production. In contrast, mice constitutionally expressing neoself-antigens did not develop the autoimmune disease because the neoself-reactive T cells were in a state of tolerance to neoself.
These findings indicate that the presentation of neoself antigens on MHC causes an autoimmune disease. In fact, a comprehensive analysis of T-cell receptors (TCRs) in patients with SLE via single-cell analysis revealed that neoself-reactive T cells were prominently expanded in these patients, as observed in mice in which neoself-antigen presentation was induced. The percentage of neoself-reactive T cells reached as high as 10% of activated T cells, indicating their central role in the pathogenesis of SLE. Thus, neoself antigens were identified as the causative molecules of a systemic autoimmune disease.
Mori said, "For example, even after an injection of a coronavirus disease vaccine, the percentage of coronavirus-reactive T cells is 1/10 of a few percent. So, 10% was a very shocking number."
Although most adults are persistently infected by the Epstein-Barr (EB) virus, the frequency of viral reactivation varies from individual to individual. A high frequency of EB virus reactivation is a risk factor for developing various autoimmune diseases.
In this study, EB virus reactivation was found to cause downregulation of the invariant chain expression and functional abnormality of MHC, resulting in the neoself antigen presentation on MHC. Furthermore, neoself antigen presentation induced by EB virus reactivation was shown to activate self-reactive T cells in patients with SLE, triggering autoimmunity. These findings indicate that T cells have the ability of "self-neoself discrimination" in addition to the previously established "self-nonself discrimination." In autoimmune diseases, self-neoself discrimination by T cells was found to induce immune responses against neoself antigens, which are self-molecules, resulting in the induction of autoimmune responses.
The study also identified, as a factor inducing neoself-antigen presentation, the reactivation of the EB virus, a persistent infection virus, triggering autoimmune responses through the expression of neoself-antigens.
Until now, the only option for autoimmune diseases has been symptomatic treatment with long-term medication. The pathogenic mechanism of autoimmune diseases revealed in this study is expected to facilitate the development of radical treatments targeting the pathogenic mechanism.
Journal Information
Publication: Cell
Title: Neoself-antigens are the primary target for autoreactive T cells in human lupus
DOI: 10.1016/j.cell.2024.08.025
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




