A research group led by Associate Professor Shin Takemoto and Professor Hiroyuki Matsuzaka of the Graduate School of Science at Osaka Metropolitan University announced that they developed a catalyst that can selectively synthesize biopropylene under mild conditions close to room temperature and pressure. They successfully achieved the highly efficient synthesis of propylene from glycerin, a byproduct of the biodiesel production process. This work is expected to contribute to a sustainable chemical industry. The results were published in the international journal Chemical Communications on August 6.
A new catalyst can selectively reduce allyl alcohol, leading to a bio-based propylene.
Provided by Shin Takemoto, Osaka Metropolitan University
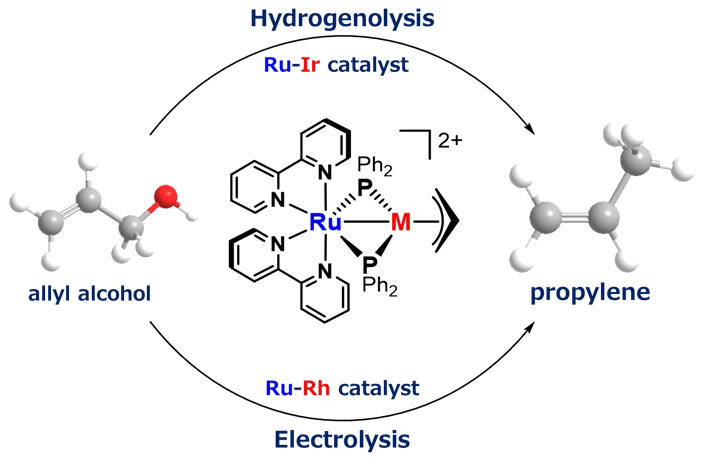
Propylene is made from petroleum and is widely used as a raw material for many plastics and chemical products. Glycerin is a byproduct generated in large quantities when producing biodiesel from vegetable oils, and a useful substance derived from glycerin is allyl alcohol. Allyl alcohol is a molecule with a double bond (C=C bond) and an oxygen bond (C-O bond). Propylene can be produced from biomass if only the oxygen bond can be selectively reduced; however, realizing this has been difficult because the double bond is usually more reactive.
In these developments, the research group developed a unique catalyst for the selective reduction of allyl alcohol to propylene. The catalyst contains a special molecule called a metalloligand, which is designed to reversibly bind two metals in the catalyst. The C-O bond of allyl alcohol obtained from glycerin (waste oil) was selectively hydrogenolyzed under conditions close to room temperature and pressure, and propylene was successfully synthesized with high efficiency.
Takemoto said, "In this research, we were able to create a new catalyst molecule and develop a technology that contributes to the carbon neutrality of sustainable propylene production. In the future, we intend to further develop this technology and conduct research toward its practical application."
Journal Information
Publication: Chemical Communications
Title: Bimetallic Ru-Ir/Rh complexes for catalytic allyl alcohol reduction to propylene
DOI: 10.1039/D4CC01711K
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




