Graduate Students Yukino Ofuchi and Sae Doi (at the time of research), both members of Professor Yasushi Sekine's research group at Waseda University's Faculty of Science and Engineering, and Researcher Kenta Mitarai at Yanmar Holdings, have discovered a new hydrogen production method using clean ammonia decomposition. By employing a new catalytic process to extract hydrogen from ammonia, which is expected to be used as one of the hydrogen carriers for hydrogen storage and transportation, the reaction temperature was successfully lowered to approximately 200℃, 200℃ lower than the conventional temperature (400℃). It was also revealed that this novel catalytic process has a different mechanism than the conventional ammonia decomposition reaction.
Provided by Waseda University
The use of hydrogen as a clean energy source toward realizing a carbon-free society is progressing, but its storage and transportation are difficult. This has resulted in the use of hydrogen carriers that can efficiently store and transport hydrogen being considered, and ammonia is garnering attention as one of these.
Ammonia is one of the most widely synthesized compounds in the world. Its high hydrogen content of 17.6 wt% and easy liquefaction and portability make it a strong candidate for a hydrogen carrier. However, when it is used as a hydrogen carrier, an ammonia decomposition reaction is required to eventually extract the involved hydrogen. This reaction is more likely to occur at higher temperatures, and a high temperature of 400℃ or higher is generally required to decompose 100% of ammonia. It is essential to lower the temperature of the ammonia decomposition reaction to obtain hydrogen more efficiently and conveniently.
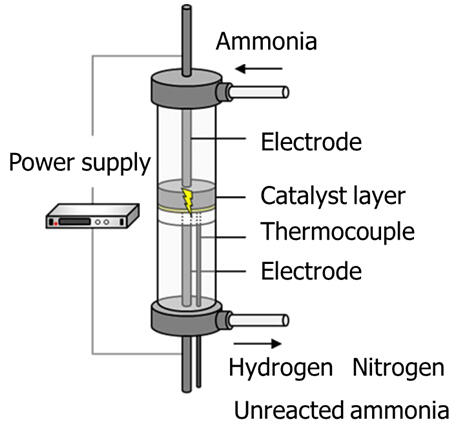
In this research, the group used an electric field-assisted catalytic reaction that promotes various reactions to extract hydrogen at low temperatures. This is a process in which electrodes are brought into direct contact with the catalyst layer from above and below and an electric current is passed through them to accelerate the reaction. The catalyst used was cerium oxide (CeO2), which exhibits semiconducting properties, with the precious metal ruthenium (Ru) and base metals such as iron (Fe), nickel (Ni), and cobalt (Co) on the top.
As a result, the electric field-assisted ammonia decomposition reaction with an electric current achieved an ammonia decomposition rate of approximately 100% at 125℃, a low temperature where the reaction would not progress to this extent in the past. It was also found that the reaction was greatly accelerated at low temperatures when the expensive Ru was used as the catalyst as well as when inexpensive metals such as Fe and Ni were used. Furthermore, in the electric field-assisted ammonia decomposition reaction, a peculiar phenomenon was observed in a temperature range of 100℃-200℃, where the reaction rate increases with decreasing temperature, unlike for conventional reactions.
Based on these results, the research group believed that a new reaction mechanism involving adsorption phenomena is occurring, which is more favorable at lower temperatures. They conducted various experiments to understand the mechanism. The results revealed that in the conventional reaction mechanism, nitrogen desorption was promoted from the catalyst surface. This desorption is the "rate-limiting step" of the elementary reactions in a given chemical reaction; it corresponds to slowest progression and determines the overall reaction rate among the elementary reactions that make up that reaction.
In the conventional reaction mechanism, hydrogen (H) is first desorbed from ammonia (NH3) and nitrogen (N) remains on the catalyst surface. The involved N atoms then bond with each other to produce nitrogen molecules (N2), but this process requires the most energy to proceed and is the reason why the ammonia decomposition reaction requires high temperatures. In contrast, in the method used herein, when an electric current is applied to the catalyst, the catalyst surface becomes rich in hydrogen ions, increasing the amount of NH, which comprises one nitrogen and one hydrogen. It was inferred that the reaction mechanism involves the generation of an intermediate substance called N2H2 through the bonding of N and H, which is easier than the bonding of N atoms, and the completion of the ammonia decomposition reaction through the desorption of hydrogen from this intermediate substance.
The research group examined this mechanism in detail in a theoretical simulation using machine learning potential. As a result, it was found that the mechanism that uses the intermediate substance N2H2 tends to proceed at the interface between Ru and CeO2 during the electric field-assisted reaction, which is a promising mechanism for the electric field-assisted ammonia decomposition reaction to proceed at low temperatures.
The group expressed the following expectation: "the success of the ammonia decomposition reaction with a high decomposition rate at low temperatures will make it possible to obtain hydrogen on site using small facilities by utilizing waste heat from factories, engines, etc. and lead to the expansion of ammonia use as a hydrogen carrier."
Journal Information
Publication: Chemical Science
Title: Hydrogen production by NH3 decomposition at low temperatures assisted by surface protonics†
DOI: 10.1039/d4sc04790g
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




