Yokogawa Bridge Co. (Funabashi City, Chiba Prefecture), Kobe University, and Toagosei Co., Ltd. (Minato City, Tokyo Prefecture) have jointly developed a sheet that can remove chlorine ions, the source of rust, by applying it to areas where damage by rust is severe and then peeling it off during bridge repainting. This simple application prevents deterioration of the paint due to residual salt, which is expected to shorten maintenance time. Professor Minoru Mizuhata, who studies Inorganic Materials Chemistry at Kobe University came up with the idea from medical patches, and its commercialization is currently under consideration.

Provided by Yokogawa Bridge
According to Satoshi Maeda, assistant manager (bridge engineering) of the Yokogawa Bridge Holdings Technical Research Laboratory, which is involved in bridge construction, bridges made of steel and other materials typically require regular repainting work approximately every 20-30 years to prevent corrosion of the involved components. However, bridges in coastal areas with high levels of airborne salt, those in cold regions where antifreeze is sprayed in winter, and bridge girders where water tends to accumulate are prone to rusting. If they are repainted without sufficient salt removal, repeated corrosion will occur soon after repainting.
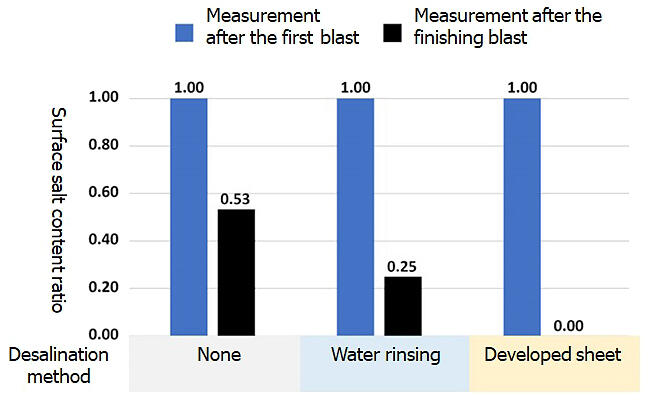
When repainting, high-pressure water rinsing is effective, but in areas where drainage treatment is difficult, "blast treatment" - i.e., spraying iron powder and scraping the surface of the rusted area - is performed to remove the chlorine ions that cause rust. However, there is a problem that the process must be repeated, which is time consuming and costly.

Provided by Yokogawa Bridge
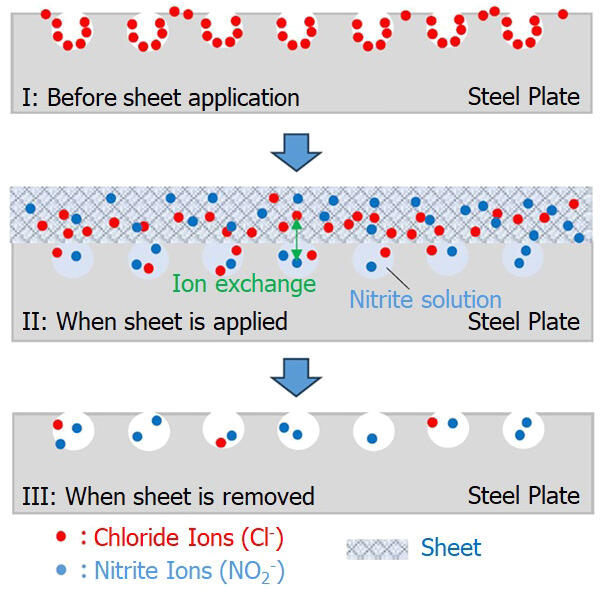
As the deterioration of steel bridges built during the bubble period was becoming an issue, Yokogawa Bridge consulted Professor Mizuhata of the Department of Chemical Science and Engineering, Graduate School of Engineering at Kobe University, around 2018. The purpose of this consultation was to see "if it was possible to create a rust prevention sheet that is simpler and lasts longer than conventional sheets." Conventional sheets prevent rusting based on the substances they contain, but they do not remove the chlorine ions that are the source of rust. While thinking about the need to apply a soft gel absorbed with liquid to penetrate the medicine into the uneven surface of the rust, Mizuhata came up with the idea of using a compress.
Mizuhata received the materials for the compress from Toagosei, a chemical products manufacturer with whom he was collaborating on another matter. He created a gel material containing nitrite ions, which has the effect of preventing rust, and a gel material containing layered double hydroxide (LDH), which exhibits an ion-exchange function upon nitrite ion addition. When a bridge girder that had rust on it was cut out and blast-treated, the compress was attached to it, and then peeled off, no rust was observed on it for approximately a month.

Provided by Yokogawa Bridge
The difference from conventional sheets is that chlorine ions can be removed when the applied sheet is removed. Because it contains LDH with an anion-exchange function, the involved mechanism is such that chlorine ions that are in the vicinity of the rust move by diffusion into the gel containing water. If the sheet is removed, the chlorine ions are removed with the gel. Nitrite ions remain on the steel plate to enhance the rust-preventive effect.
Provided by Yokogawa Bridge
Provided by Yokogawa Bridge
The researchers are considering commercialization of the developed sheet, but in the future, they will conduct test constructions to determine its ease of use on-site and its size. Maeda said, "We are developing this product on the assumption that it will prevent rust occurrence on bridges, but there may be a need for it in plants and other buildings as well. We would be happy if it could be widely used."
The results were orally presented on September 5 at the 79th Annual Conference of the Japan Society of Civil Engineers held at Tohoku University.
Original article was provided by the Science Portal and has been translated by Science Japan.




