A research group led by Assistant Professor Shouta Nonoyama and Professor Shinji Masuda of the Department of Life Science and Technology at Tokyo Institute of Technology (currently at the Institute of Science Tokyo), in collaboration with Professor Kan Tanaka at the Laboratory for Chemistry and Life Science, Institute of Innovative Research of the same Institute and Assistant Professor Yasuhiro Gotoh (currently at the National Institute of Genetics) and Professor Tetsuya Hayashi at the Faculty of Medical Sciences, Kyushu University, has announced their research results showing that the ability of bacteria to synthesize hydrogen sulfide (H2S) is involved in the regulation of iron (Fe) uptake activity. They analyzed mutant Escherichia coli with excessive hydrogen sulfide synthesis capacity. They also found that the loss of this regulation resulted in decreased antibiotic resistance. The findings are expected to contribute to developing antibiotics that do not cause drug resistance. The results were published in the international journal mBio on September 26.
Provided by Institute of Science Tokyo
Fe is an essential element for life, and bacteria have various mechanisms to efficiently take up iron. In particular, intestinal bacteria compete with host organisms since mammals' intestinal environments tend to be iron-deficient. For example, intestinal bacteria synthesize and secrete iron-binding organic molecules called siderophores for efficient iron uptake.
Meanwhile, mammals are known to prevent infection by pathogenic bacteria by inhibiting siderophore function. Iron has also been reported to be involved in antibiotic resistance, host infection, and growth via reactions with hydrogen sulfide, which is produced at certain levels in bacteria.
Previously, Masuda and his colleagues had successfully identified SqrR, a hydrogen sulfide-responsive transcription factor in photosynthetic bacteria. This transcription factor is also conserved in intestinal bacteria that do not actively utilize hydrogen sulfide. Hydrogen sulfide in intestinal bacteria was thought to be involved in transcriptional regulation, but the details were unknown.
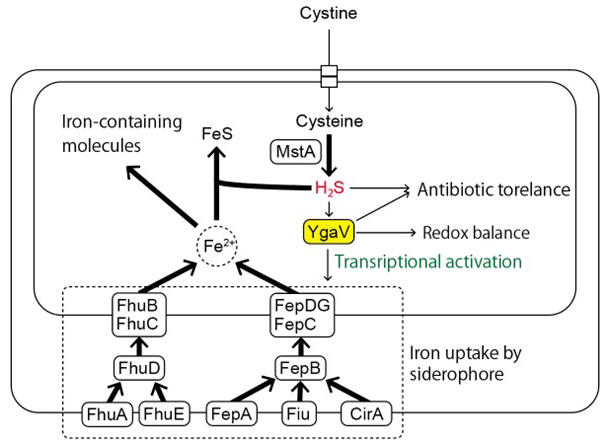
In this study, the research group investigated the function of hydrogen sulfide synthesis in Escherichia coli, a representative example of intestinal bacteria. Specifically, they generated E. coli overexpressing MstA, an enzyme for hydrogen sulfide synthesis, and examined changes in gene expression through comprehensive transcript analysis. The results showed the increased expression levels of genes related to the formation of siderophores (such as FepA and CirA) and proteins responsible for siderophore uptake (such as EhuB/C and FeC/D/G) were upregulated. Overexpression of MstA in an E. coli (normally anaerobic) mutant of "YgaV," a protein functionally similar to SqrR (homolog), did not result in such increased transcription.
These analyses revealed that "YgaV" detects hydrogen sulfide synthesized in the cells and activates the expression of genes required for iron uptake according to the intracellular hydrogen sulfide level. Highly toxic hydrogen sulfide is synthesized from cysteine (an amino acid) by MstA, the amount of which appears to be tightly controlled. The present study revealed a mechanism where an increased intracellular hydrogen sulfide concentration induces increased expression of genes related to uptake of extracellular iron by "YgaV," and an increased iron content then induces the activation of reactive oxygen species scavenging enzymes and increased antibiotic resistance. "YgaV" has been shown to reduce the production of reactive oxygen species by regulating the expression of genes associated with anaerobic respiration. It is involved in antibiotic resistance and the maintenance of redox (oxidation-reduction) balance.
Suppressing "YgaV" could prevent antibiotic resistance. Since many bacteria, like E. coli, have enzymes to synthesize hydrogen sulfide using cysteine as a substrate, like E. coli, and proteins similar to YgaV, it is highly likely that similar systems are conserved in these bacteria.
Masuda said, "This study revealed that YgaV deficiency resulted in reduced antibiotic resistance, and I believe that developing inhibitors of this transcription factor as drugs will be a direction of future research. It has not been completely clear how YgaV's function as a transcription factor is regulated by hydrogen sulfide. We hope to clarify this mechanism in the future."
Journal Information
Publication: mBio
Title: Increased intracellular H2S levels enhance iron uptake in Escherichia coli
DOI: 10.1128/mbio.01991-24
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




