A research group at the Graduate School of Medical Life Science at Yokohama City University (YCU), including Graduate Student Katsuya Takahashi, Professor Jeremy Tame, Professor Tomohiro Nishizawa, and Assistant Professor Yongchan Lee, in collaboration with Aarhus University (Denmark), the University of Nebraska (USA), and Professor Genji Kurisu and Assistant Professor Akihiro Kawamoto of the Institute for Protein Research at Osaka University, has used cryo-electron microscopy single particle analysis to reveal the molecular structure of alligator hemoglobin. The new results explain its unique allosteric regulation by bicarbonate ion binding, that allows crocodilians (alligators and crocodiles) to stay underwater for extended periods. These findings are expected to contribute to our understanding of the evolution of fundamentally important proteins, such as hemoglobin. The results were published in the international journal Nature Communications on August 2.
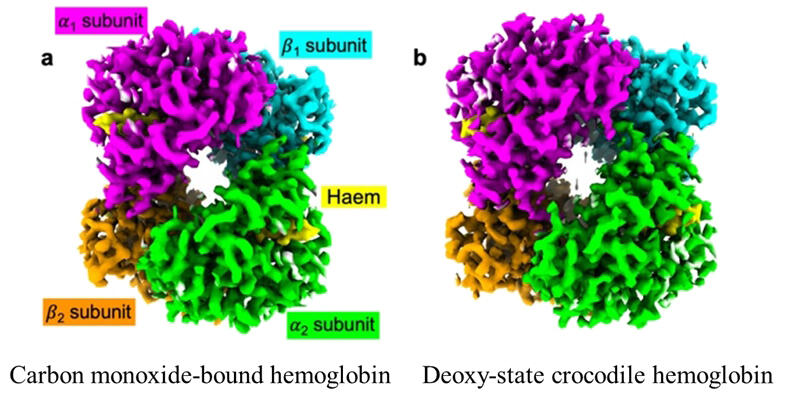
b) cryo-EM density map of the deoxy-state crocodile Hb.
The α1, β1, α2, and β2 subunits are shown in magenta, cyan, lime, and orange, respectively. The heme groups are shown in yellow.
Provided by YCU
Hemoglobin is an oxygen-transporting protein in the blood of vertebrates. It has a tetrameric structure consisting of two α-subunits and two β-subunits. Each subunit includes a reddish iron-containing small molecule called heme, to which one molecule of oxygen can bind. The hemoglobin tetramer assumes a so-called R (Relaxed) state when it binds oxygen, and a T (Tense) state when oxygen is removed. These structural changes of most vertebrate hemoglobins are allosterically regulated by organophosphates present in the red blood cells.
Allosteric regulation is a mechanism in which oxygen binding to one subunit of hemoglobin induces structural changes of other subunits of the tetramer, increasing their affinity for oxygen. Through this mechanism, oxygen binding and hemoglobin dissociation occur in a coordinated manner. Hemoglobin from alligators and crocodiles has been known for more than 40 years to be strongly regulated by bicarbonate ions, and these animals remain the only ones known with this mechanism. Owing to the bicarbonate effect, crocodiles can stay underwater for a long time. However, structural analysis by X-ray crystallography, to understand the binding of bicarbonate ions, has been impossible because suitable crystals of crocodile hemoglobin could not be obtained.
In this study, the research group attempted to determine the structure of alligator hemoglobin by cryo-electron microscopy single particle analysis. As a result, the research group successfully revealed the molecular structures of three different states, namely oxyhemoglobin, carbonmonoxy-hemoglobin, and deoxyhemoglobin (oxygen-free). Furthermore, in the molecular structure of the deoxy state, two bicarbonate ions were found at interfaces between the α and β subunits of the hemoglobin tetramer. These binding sites are different from those of organophosphates in hemoglobin of other vertebrates, and are unique to hemoglobins from crocodilians. The bicarbonate ion binding site is made by 8 amino acids. Two of these in the β-subunit were found to be particularly important for hemoglobin regulation by bicarbonate: lysine at position 38 (usually threonine) and tyrosine at position 41 (usually phenylalanine). It is hoped that in the future these findings will have medical importance by helping to develop artificial blood for transfusion.
Nishizawa said, "The unique properties of alligator hemoglobin were reported more than 40 years ago, but the details were not clarified. After moving to Yokohama City University and setting up a laboratory, I met Prof. Tame, a hemoglobin expert. We talked about a project to analyze samples by cryo-electron microscopy. This is how our collaboration started. Finally, I am very pleased that our cryo-electron microscopy analysis technique led to these interesting results."
Journal Information
Publication: Nature Communications
Title: The unique allosteric property of crocodilian haemoglobin elucidated by cryo-EM
DOI: 10.1038/s41467-024-49947-x
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




