Improved activity is essential for the practical application of water-splitting photocatalysts, which can produce hydrogen gas by exposing water to sunlight. Water-splitting photocatalysts consist of a photocatalyst host and a cocatalyst. Although there have been many reports on the improvement of photocatalyst hosts, there is still room for improvement of cocatalysts, which provide the actual reaction sites.
The research group of Professor Yuichi Negishi of the Institute of Multidisciplinary Research for Advanced Materials at Tohoku University; Junior Associate Professor Tokuhisa Kawawaki and Master's Students Daisuke Hirayama and Sota Oguchi of Tokyo University of Science; and Akihiro Higami of Mitsubishi Materials Corporation has succeeded in developing a new method for selectively loading the ultrafine rhodium-chromium mixed-oxide (Rh2-xCrxO3) cocatalyst with particle sizes of approximately 1 nm onto a water-splitting photocatalyst, which leads to hydrogen production when water is exposed to sunlight. As a result, the researchers succeeded in achieving a water-splitting photocatalytic activity of 2.6 times higher than that achieved through conventional cocatalyst loading methods. These results are published in Journal of the American Chemical Society.
©Yuichi Negishi et al.
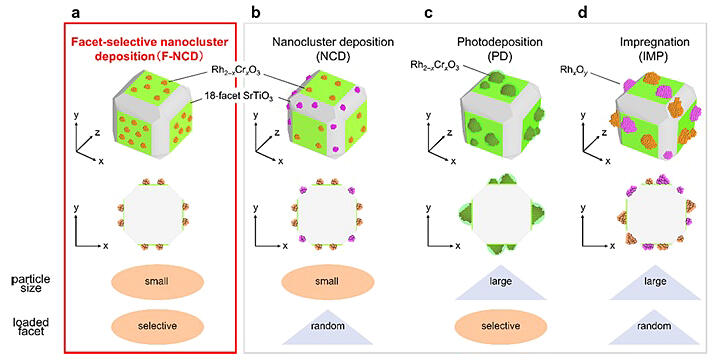
The practical application of water-splitting photocatalysts requires an increase in the solar-to-hydrogen conversion efficiency (STH) from the current value of 1.1% to 5%-10%. Cocatalysts (fine metal or metal-oxide particles) act as reaction sites and play an important role in hydrogen production by facilitating charge separation. Further, their high functionality is extremely effective in improving the activity of photocatalysts. Cocatalysts are loaded onto photocatalysts by photoelectrodeposition (PD) or impregnation (IMP) methods. Although these methods are simple, it is very difficult in principle to precisely control the size and electronic structure of cocatalysts. When a cocatalyst is loaded as ultrafine particles, the specific surface area of the cocatalyst increases, greatly improving the activity of the photocatalyst per unit amount of the loaded cocatalyst.
The research group previously developed the NCD method, which precisely synthesizes ultrafine metal nanoclusters (NCs) with a particle size of approximately 1 nm and then loads the NCs on a photocatalyst with their original particle size. Some photocatalysts have crystal facets that facilitate the migration of excited electrons and holes. By loading appropriate hydrogen- and oxygen-generating cocatalysts on these facets through which electrons and holes can easily migrate, efficient space charge separation is achieved, promoting the efficiency of each reaction and further improving the activity of water-splitting photocatalysts.
However, in the NCD method, a cocatalyst is nonselectively loaded on each crystal facet. Thus, the cocatalyst is loaded even on crystal facets where the desired reaction does not occur. Rh2−xCrxO3 particles are known to act as highly active hydrogen-production cocatalysts because they not only induce a high hydrogen-production rate but also inhibit the reverse reaction. The research group created a crystal-facet-selective nanocluster loading method (F-NCD) to selectively load ultrafine Rh2−xCrxO3 particles with sizes of approximately 1 nm on the hydrogen-producing facets of a photocatalyst host (18-facets of strontium titanate).
Specifically, two improvements were made to the NCD method reported previously by the group. The chemisorption of a cocatalyst precursor (Rh complex) on oxygen-producing surfaces was suppressed by adding an organic substance that protected certain crystal facets. The introduction of photoreductive ligands facilitated the immobilization of the adsorbed Rh complexes on hydrogen-producing crystal facets, increasing the adsorption rate of the Rh complexes.
The photocatalysts prepared by the F-NCD method were compared with those prepared by loading cocatalysts using the IMP and other methods. The results showed that the proposed method achieved a 32% finer particle size, 88% crystal-facet selective loading (57% achieved by the PD method; 2.6 times higher loading than the PD method), and 14 times higher water-splitting photocatalytic activity. The F-NCD method can also be used for other photocatalysts. Doping strontium titanate photocatalysts with different metals can improve their activity and make them responsive to visible light. It is anticipated that by combining these with the F-NCD method in the future, a large number of highly efficient water-splitting photocatalysts can be developed that can accelerate the transition to a hydrogen-energy-based society.
Journal Information
Publication: Journal of the American Chemical Society
Title: Ultrafine Rhodium-Chromium Mixed-Oxide Cocatalyst with Facet-Selective Loading for Excellent Photocatalytic Water Splitting
DOI: 10.1021/jacs.4c07351
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




