A research group led by Assistant Professor Shinsuke Uchikawa and Associate Professor Tomokazu Kawaoka of the Department of Gastroenterology at Hiroshima University has announced their results showing that tumor markers are useful in evaluating the response to "durvalumab plus tremelimumab combination therapy," a systemic drug therapy using immune checkpoint inhibitors, in patients with hepatocellular carcinoma. The tumor markers were also found to be useful in detecting "pseudo-progression," in which the tumor appears to be swollen in computed tomography (CT) and other imaging evaluations while the inhibitors are effective. In addition, they identified cut-off values for tumor markers that can be used to distinguish responders and non-responders at an early stage of treatment. The findings are expected to contribute to the establishment of a less burdensome response evaluation method for patients. The results were published in the international journal Hepatology Research on August 17.
Provided by Hiroshima University Hospital
Immune cells have an immune checkpoint function that acts as a brake to prevent the immune systems from falsely attacking healthy cells. Some cancer cells use immune checkpoints as a brake in the immune responses against cancer cells. Immune checkpoint inhibitors release this brake so that immune cells can properly attack cancer cells, and they have recently become available for hepatocellular carcinoma. Meanwhile, immune checkpoint inhibitor therapy can cause "pseudo-progression," in which the tumor appears to be swollen in CT, magnetic resonance imaging (MRI), and other imaging evaluations. In contrast, the tumor size has decreased, indicating treatment effectiveness. Thus, an index to determine the treatment response has been awaited.
Tumor markers are special substances produced by cancer or normal cells in response to cancer and are used as diagnostic indices. To explore such tumor markers, the research group analyzed 33 patients who underwent dual immune checkpoint inhibitor therapy with durvalumab and tremelimumab for hepatocellular carcinoma between April and December 2023.
The tumor markers AFP, DCP, and AFP-L3 fractions were measured before treatment and at 1, 4, and 8 weeks after the start of the treatment. CT and MRI scans were also performed 4 and 8 or 12 weeks after initiation of treatment to observe how the cancer changed. The results showed that, even if the tumors appeared to be larger on CT and MRI at 4 weeks, the values of the 3 tumor markers were significantly decreased at 8 weeks in the responder group of patients. In contrast, the DCP value was increased at 4 weeks in the non-responder group.
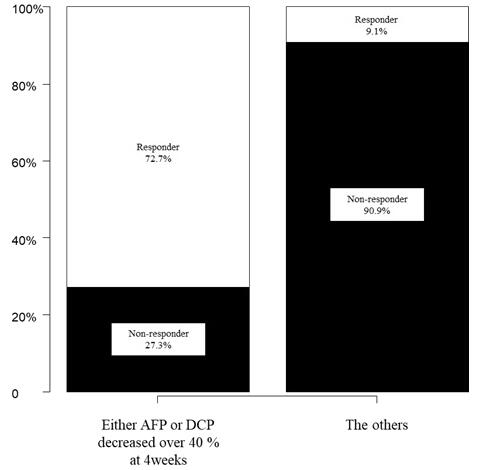
A cut-off value to predict the effectiveness of treatment was determined as a decrease of at least 40% in tumor markers at 4 weeks. They confirmed that 72.2% of the patients in whom the tumor marker AFP or DCP decreased at least 40% at 4 weeks were responders. Changes in the tumor markers are expected to allow for more accurate treatment response evaluation than CT or MRI, without the risk of radiation exposure and contrast-induced nephropathy that can occur during these imaging tests.
Kawaoka said, "In this study, we showed that the combination of two tumor markers can predict the effectiveness of durvalumab plus tremelimumab for unresectable hepatocellular carcinoma. I hope that these data will be helpful in decision-making regarding treatment switching in clinical practice. I also believe this rule can be used in treatment with novel drugs in the future."
Journal Information
Publication: Hepatology Research
Title: Significance of changes in tumor markers in patients treated with durvalumab plus tremelimumab combination therapy as a surrogate marker for tumor response to unresectable hepatocellular carcinoma
DOI: 10.1111/hepr.14104
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




