A research group led by Professor Eijiro Miyako of the Materials Chemistry Frontiers Research Area, Graduate School of Advanced Science and Technology, Japan Advanced Institute of Science and Technology (JAIST) has announced that they successfully produced nanoparticles by coating the surface of carbon nanohorns (CNHs), a type of carbon nanotubes, with cancer cell components and an anticancer drug and demonstrated that they exhibit not only enhanced permeability and retention effects unique to nanoparticles but also various cancer treatment effects in mice. The nanoparticles had marginal effects on the body, and the combined use of the nanoparticles and near-infrared laser light is expected to develop into a new cancer theranostic platform. The results were published in the international journal Small Science on August 19.
Provided by JAIST
It is known that cancer cells have specialized cell membrane functions to evade attacks from immune cells and that components in cancer cells (such as genes and proteins) enhance immune activity. Miyako previously discovered that CNHs not only have high biocompatibility and excellent physicochemical properties but also easily generate heat with laser light in the wavelength range (650-1100 nanometers) that is highly permeable to living tissue. He has been developing cancer diagnosis and treatment technologies utilizing these properties.
Meanwhile, due to strong intermolecular interactions, CNHs are difficult to disperse in water and other media, and their tendency to aggregate has been an issue. A method to eliminate aggregation is to chemically modify the surface with a water-soluble polymer such as polyethyleneglycol. However, the polymer-modified CNHs have problems with loss of blood retention and allergic reactions after repeated administration.
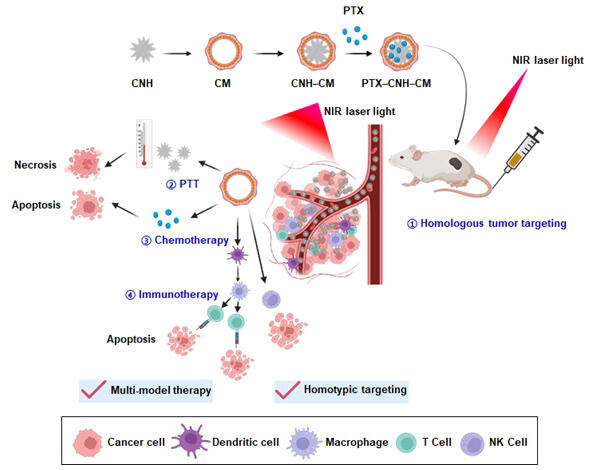
With the technology developed in this study, cancer cell components are adsorbed on the CNH surface by simple ultrasonic irradiation so that CNHs can be dispersed in an aqueous solution. Moreover, by utilizing cancer cell components, they also succeeded in simultaneously coating the CNH surface with "paclitaxel (PTX)," an anticancer drug insoluble in water. The cancer cell component-PTX-CNH complex prepared by this method was confirmed to have high membrane permeability and anticancer activity against cells, with a particle size stability of at least 30 days. The research group also confirmed heat generation by near-infrared laser light irradiation. Thus, they tested the CNH complex in the visualization of cancer lesions and its therapeutic effectiveness.
The visualization of cancer lesions uses nanoparticles (cancer cell component-ICG-CNH complex) produced by binding the near-infrared fluorescent dye "indocyanine green (ICG)," which can be used for cancer diagnosis, to the CNH surface together with cancer cell components. When the cancer cell component-ICG-CNH complex was administered to mice about 10 days after colon cancer transplantation and exposed to near-infrared light (740-790 nm) 24 hours later, the images showed that the cancer-affected areas emitted fluorescence.
When the affected area where the cancer cell component-ICG-CNH complex was accumulated was irradiated with 808-nm near-infrared laser light, complete elimination of cancer was successfully achieved after 2 days via the photothermal conversion effect of CNH in addition to the blood retention, intratumoral infiltration, and immune activation effects from the cancer cell components and the pharmacological effects from the anticancer drug. Further investigation of the mechanism of drug action in tumors revealed that immune cells such as cytotoxic T cells and natural killer cells were activated by the laser-irradiated cancer cell component-ICG-CNH complex.
Miyako said, "We expect that this technology provides an effective and precise approach to cancer treatment, particularly to the treatment of metastatic cancer, since it is a single platform integrating the benefits of multiple therapeutic modalities, such as targeted cancer therapy, photothermal therapy, chemotherapy, and immunotherapy. Currently, we are also vigorously conducting safety and other studies to conduct clinical trials within 10 years."
Journal Information
Publication: Small Science
Title: Biomimetic Functional Nanocomplexes for Photothermal Cancer Chemoimmunotheranostics
DOI: 10.1002/smsc.202400324
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




