One of the causes of Alzheimer's disease is abnormal accumulation of amyloid β (Aβ) in the brain. Aβ molecules assemble into clumps called fibrils, which adversely affect neurons. Although blocking the growth of Aβ fibrils is considered important for the treatment of Alzheimer's disease, the growth process and mechanism of growth cessation were not fully understood.
A research team led by Professor Koichi Kato of the Exploratory Research Center on Life and Living Systems and the Institute for Molecular Science, National Institutes of Natural Sciences (Professor of the Graduate School of Pharmaceutical Sciences, Nagoya City University), Professor Takayuki Uchihashi of the Graduate School of Science, Nagoya University, and the Research and Development Center for Precision Medicine, the University of Tsukuba, used state-of-the-art high-speed atomic force microscopy (HS-AFM) to observe how Aβ fibrils grow in real time at the molecular level.
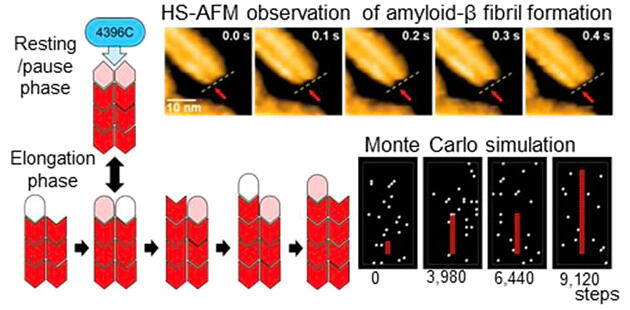
Provided by Kato et al.
The results revealed that one Aβ fibril consists of two protofilaments and that the fibrils are elongated by the alternating addition of Aβ molecules to the protofilaments. Furthermore, the pause phase, in which fibril growth temporarily stops, occurs frequently during the even edge state, in which the ends of the two protofilaments are aligned. This pause phase is an important step that occurs spontaneously during the Aβ fibril growth process. They also showed that a specific antibody called 4396C selectively binds to the edge of the fibrils in the pause state, effectively preventing further fibril elongation.
This study revealed a mechanism by which the antibody completely stops the Aβ fibril growth and prevents progression. It also showed a unique mechanism for the Aβ fibril growth through cycles of alternating elongation and pauses. These findings revealed a new element involved in the progression of Alzheimer's disease and may contribute particularly to the development of therapeutic strategies targeting Aβ fibrils in the pause state.
Moving forward, further elucidation of the mechanism of action of the 4396C antibody is expected to lead to new therapies aiming at inhibiting the growth of Aβ fibrils. Based on these findings, the research team has planned to continue their research with the aim of applying the therapeutic strategies to other forms of amyloidosis. The results were published in the Journal of the American Chemical Society.
Journal Information
Publication: Journal of the American Chemical Society
Title: Single-Molecule Kinetic Observation of Antibody Interactions with Growing Amyloid β Fibrils
DOI: 10.1021/jacs.4c08841
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




