A research group led by Principal Investigator Shoi Shi and Researcher Yusuke Iino of the International Institute for Integrative Sleep Medicine at the University of Tsukuba, in collaboration with Project Professor Haruo Kasai of the International Research Center for Neurointelligence at the University of Tokyo, Team Leader Taro Toyoizumi of the RIKEN Center for Brain Science, and other researchers, has announced that they have developed a new molecular tool called "SYNCit-K," which increases synaptic strength, and a mathematical model called the "EIN model," which predicts the relationship between synaptic strength and brain activity, with the aim of investigating the mechanism by which the quantity and quality of sleep are maintained at constant levels. Application of the tool to the mouse frontal lobe induced sleep. However, strong sleep was not induced when synaptic potentiation was inhibited. Moreover, the increased synaptic strength declined to the original level during subsequent sleep. The findings are expected to contribute to the development of treatments to improve sleep quality and were published in the international journal Science on Sep. 26.
Provided by the University of Tsukuba
In many organisms, prolonged sleep deprivation increases the need for sleep and undergo deeper sleep to maintain the overall quantity and quality of sleep. Sleep-deprived mice and mice with a genetic mutation that causes deeper sleep have been shown to have different protein statuses in synapses, which connect neurons. The conduction efficiency of synapses (synaptic strength) has synaptic plasticity, which is enhanced by learning and other means. Glutamate, which is the major information transmitter, activates the cells that received itself (postsynaptic cells). Postsynaptic cells have dendrites (spines), the sizes of which are proportional to synaptic strength. Spine strength and spine size in the cortex correlate with the need for sleep. In contrast, it was unclear whether changes in synaptic strength are caused by sleep or whether changes in synaptic strength regulate sleep in response to the need for sleep.
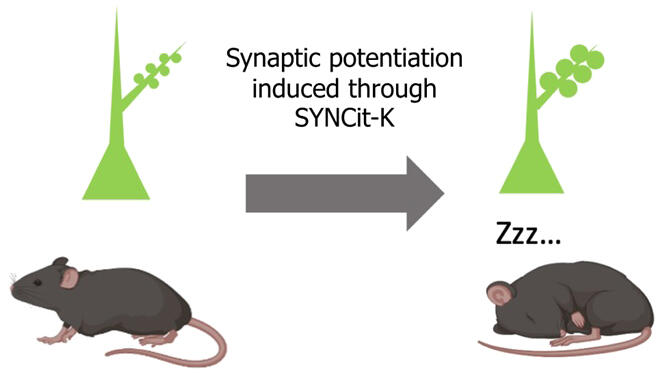
In this study, the research group developed the novel molecular tool "SYNCit-K" to induce synaptic potentiation and spine enlargement. Based on chemically induced protein dimerization, the tool causes accumulation of the enzyme Karilin7 in the spines, inducing synaptic potentiation and spine enlargement. The research group introduced this tool to individual mice to induce synaptic potentiation of excitatory neurons in the frontal lobe and found that the amount of sleep and delta power, an electroencephalographic indicator of the depth of sleep (higher values indicate deeper sleep), were increased. They also confirmed that the increased synaptic strength declined to the original level during subsequent sleep. Furthermore, inhibition of an enzyme essential for synaptic potentiation abolished the sleep deprivation-induced delta power increase.
Next, the research group developed the "EIN model," a mathematical model describing the activity of neurons in the cerebral cortex. They used the model to investigate the effects of changes in synaptic strength on the activity of neurons. The results showed that when the synaptic strength was increased, neurons exhibited sleep-like firing patterns and the delta power on electroencephalograms increased. These predictions were also consistent with the experimental results in cultured neurons and mice.
Shi said, "Sleep research has made remarkable advances in recent years, and the centuries-old mystery of 'why do living organisms sleep' is about to be solved in this century. In our study, experts in mathematics, molecular biology, and sleep research were brought together and provided a promising answer that organisms sleep to regulate the connections between neurons. We look forward to seeing how the mysteries of sleep will be unraveled in the future, as our answer is tested by researchers around the world."
Journal Information
Publication: Science
Title: Prefrontal synaptic regulation of homeostatic sleep pressure revealed through synaptic chemogenetics
DOI: 10.1126/science.adl3043
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




